视神经损伤
-
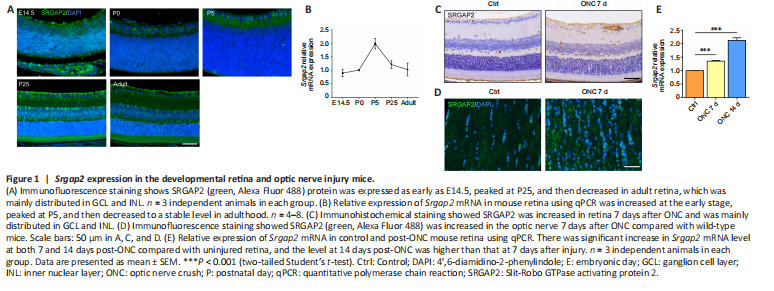
Figure 1|Srgap2 expression in the developmental retina and optic nerve injury mice.
Slit/Robo signaling has been shown to be involved in the regulation of RGC axon guidance (Erskine et al., 2000). We found increased expression of Slit2/Robo1 in the retina, and decreased expression in the optic nerve after ONC (Additional Figure 1B–E). As the downstream molecule of Slit/Robo signaling, SRGAP2 plays significant roles in the central nervous system, but its function is unclear in normal neuroretina and optic neuropathies (Fossati et al., 2016). Therefore, we first determined the expression pattern of Srgap2 during the developmental stage of retina. Immunofluorescence staining showed SRGAP2 protein expression in embryonic day 14.5, postnatal day 0 (P0), P5, P25, and adult mice, with distribution particularly in the retinal nerve fiber layer, GCL, and inner nuclear layer during retinal development (Figure 1A). qPCR confirmed that Srgap2 was expressed from E14.5 to adult retina (Figure 1B). Immunohistochemistry showed that SRGAP2 protein level was higher in the retina and optic nerve 7 days after ONC compared with that in the control group (Figure 1C and D). Srgap2 mRNA levels also were increased 7 to 14 days after ONC (Figure 1E).
Figure 2|RGC survival after ONC in Srgap2+/– mice.
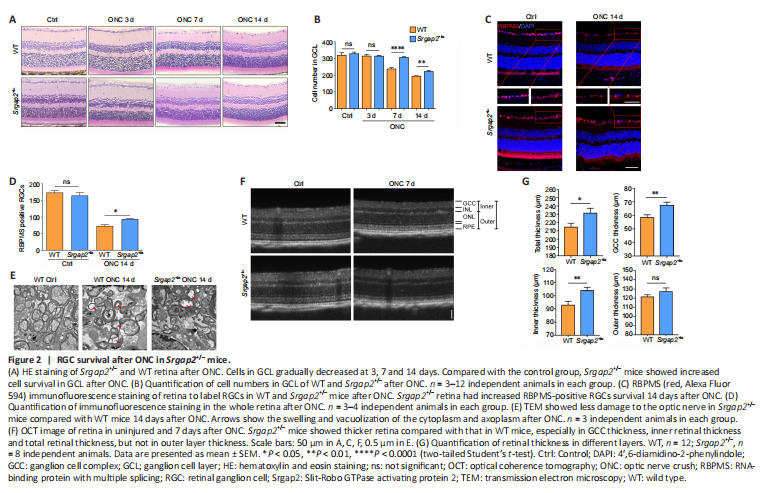
Progressive RGC loss was observed from 5–7 days after ONC. Histological analysis did not show abnormality of the retina in Srgap2+/– mice. However, after ONC, Srgap2+/– mice had increased RGC survival compared with that in WT (Figure 2A and B). RBPMS is widely used for specific labeling of RGCs (Rodriguez et al., 2014). Similar to the results of the histological analysis, there was no difference in RBPMS-positive RGCs in the uninjured retina between WT and Srgap2+/– mice, whereas more RBPMS-positive RGCs were observed in Srgap2+/– mice than in the WT group after ONC (Figure 2C and D). These results were further verified using immunostaining with antibodies against β-III tubulin (Tuj1), another neuronal marker (Zhou et al., 2021; Additional Figure 3). Transmission electron microscopy of uninjured optic nerve showed that the cytoplasm and axoplasm in the optic nerve bundle were filled with organelles, whereas ONC models had a thinner myelin sheath, swelling and vacuolization of the cytoplasm and axoplasm 14 days after ONC. These pathologic features were less apparent in Srgap2+/– mice compared with WT mice, as shown in Figure 2E.
ONC reduced the retinal thickness in mice. We used optical coherence tomography to compare retinal thickness in WT and Srgap2+/– mice in vivo and found that the total thickness, ganglion cell complex (GCC) thickness and inner thickness were thicker in Srgap2+/– mice than in WT mice 7 days post-ONC. No significant difference was observed in the thickness of the outer retinal layer between the groups (Figure 2F and G). We concluded that Srgap2 suppression provided a neuroprotective effect in the injured retina and optic nerve in vivo.
Figure 3|Glial cell distribution after ONC in Srgap2+/– mice.
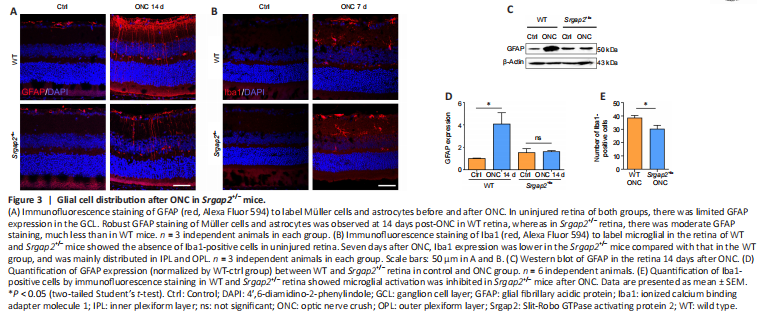
It was reported that robust activation of Müller glia and microglia was occurred after ONC (Au and Ma, 2022). Immunofluorescence staining of glial fibrillary acidic protein, a specific marker of astrocytes and activated Müller cells (Bulirsch et al., 2022), showed positive labeling only in the GCL in the uninjured retinas associated with non-activated astrocytes. However, there was robust activation of astrocytes in the GCL and Müller cells throughout the retina after ONC. Glial activation was inhibited significantly in Srgap2+/– mice compared with WT (Figure 3A). Furthermore, activation of Iba1-positive microglia in the retina after ONC was inhibited in Srgap2+/– mice (Figure 3B). We confirmed these results using western blot with antibodies against glial fibrillary acidic protein (Figure 3C and D). Quantification of Iba1-positive microglia also showed inhibition in the retina after ONC in Srgap2+/– mice (Figure 3E). These findings indicate that Srgap2 suppression ameliorated glial activation after optic nerve injury.
Figure 4|RGC survival after SO injection in Srgap2+/– mice.
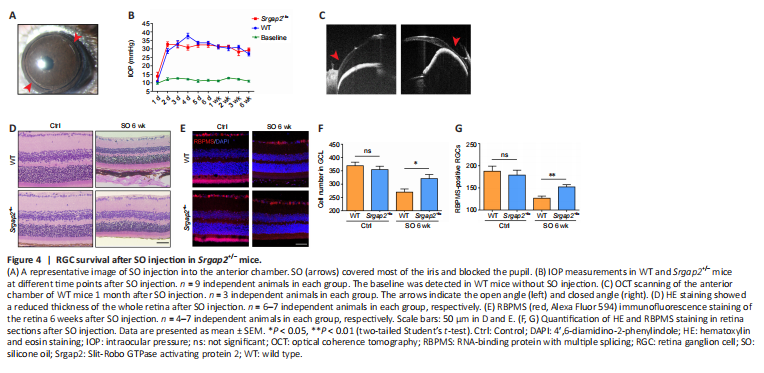
We used a second RGC injury model to verify the involvement of Srgap2 in RGC protection. SO was injected into the mouse anterior chamber, which leads to a sustained increase in IOP, resulting in chronic RGC damage (Zhang et al., 2019). IOP was elevated to 30 mmHg from 12 mmHg at baseline after SO injection and stayed at this level for 6 weeks (Figure 4A and B). Optical coherence tomography scanning showed that the anterior chamber angle was closed after SO injection (Figure 4C). Similar to the ONC results, more RGCs survived in Srgap2+/– mice compared with WT mice 6 weeks after SO injection, observed with both hematoxylin and eosin and immunofluorescence staining (Figure 4D–G). These results indicated that suppression of Srgap2 may play a protective role during neuroretinal damage.
Figure 6|Downstream signaling pathways after inhibition of Srgap2.
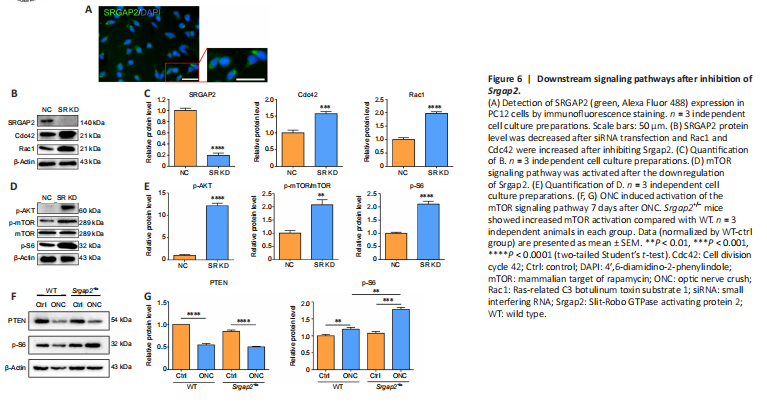
The above results indicated that Srgap2+/– mice demonstrate neuroprotective characteristics after optic nerve injury. To identify the molecular mechanisms underlying the neuroprotection, we knocked down Srgap2 in PC12 cells using RNA interference. We initially observed SRGAP2 expression in PC12 cells by immunofluorescence staining (Figure 6A). After siRNA transfection, SRGAP2 expression was reduced to about 20% of the control level in PC12. In addition, SRGAP2 suppression increased the expression levels of Cdc42 and Rac1 (Figure 6B and C), which belong to the Rho GTPase family and are downstream proteins of SRGAPs (Wong et al., 2001) that play an important role in neurotoxicity.
PI3K/AKT/mTOR signaling pathways are important for neuroprotection, and phosphatase and tensin homolog deleted on chromosome ten (PTEN) negatively regulates this pathway (Cuenca et al., 2014). We found significantly increased levels of phosphorylated AKT (p-AKT) and p-mTOR/mTOR, and increased phosphorylation of the downstream protein ribosomal s6 kinase (pS6) after Srgap2 siRNA transfection, suggesting activation of the mTOR signaling pathway (Figure 6D and E). Furthermore, PTEN expression was decreased in Srgap2+/– retina compared with WT retina after ONC (Figure 6F), and pS6 expression was increased in Srgap2+/– retina after ONC (Figure 6G). These findings indicate that Srgap2 suppression may protect the neuronal retina from damage through activation of the mTOR signaling pathway.