中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (10): 2307-2314.doi: 10.4103/1673-5374.369122
抑制Srgap2可减轻小鼠视网膜神经节细胞变性
Srgap2 suppression ameliorates retinal ganglion cell degeneration in mice
Yi-Jing Gan1, 2, Ying Cao1, Zu-Hui Zhang1, Jing Zhang1, Gang Chen1, Ling-Qin Dong1, Tong Li1, Mei-Xiao Shen1, Jia Qu1, *, Zai-Long Chi1, *
- 1State Key Laboratory of Ophthalmology, Optometry and Vision Science; Eye Hospital, Wenzhou Medical University, Wenzhou, Zhejiang Province, China; 2Department of Ophthalmology, The First Affiliated Hospital of University of Science and Technology of China, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui Province, China
摘要:
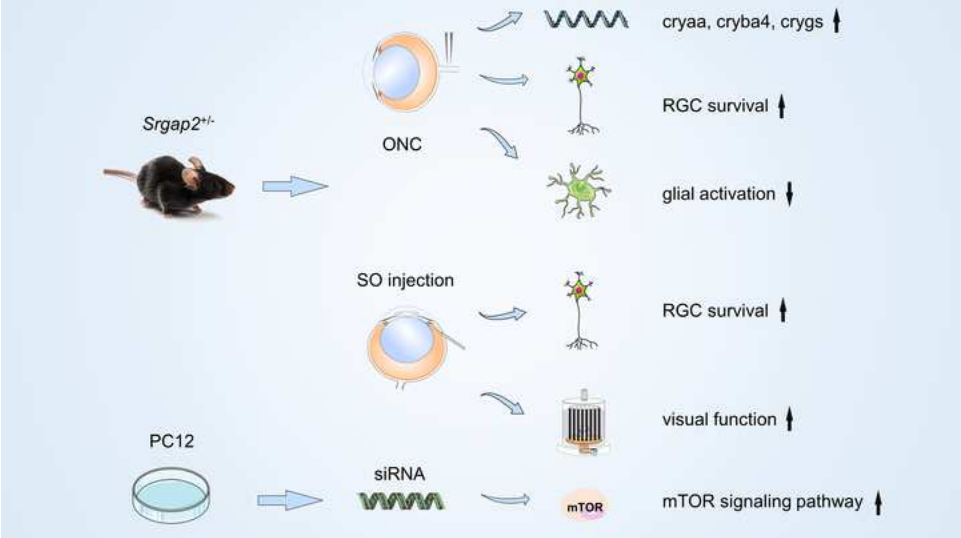
Slit-Robo-Rho-GTPase激活蛋白2(SRGAP2)在轴突引导、神经元迁移、突触形成和神经再生中发挥着重要的作用,但其在神经视网膜变性疾病中的作用尚不清楚。实验首先发现,正常小鼠视网膜在胚胎期即开始表达SRGAP2蛋白,且主要位于成熟的视网膜神经节细胞层和内核层,而视神经夹闭损伤后,小鼠视网膜和视神经中SRGAP2蛋白水平升高。进一步以Srgap2杂合子(Srgap2+/-)小鼠建立视神经夹闭模型,发现抑制Srgap2可提高视网膜神经节细胞的存活率,降低眼内压,抑制胶质细胞活化,并部分恢复视网膜功能。而体外实验中沉默Srgap2基因则能促进PC12细胞中哺乳动物雷帕霉素靶蛋白信号通路的激活。视网膜转录组测序发现,Srgap2+/-小鼠视网膜中与视神经损伤保护相关的小热休克蛋白基因(cryaa,cryba4和crygs)水平上调。上述结果表明抑制Srgap2基因表达可通过减缓胶质细胞的过度活化,并激活神经保护相关的哺乳动物雷帕霉素靶蛋白通路和小热休克蛋白的表达,改善视网膜神经节细胞变性和挽救部分视神经功能。
https://orcid.org/0000-0002-3617-6425 (Zai-Long Chi); https://orcid.org/0000-0001-5729-567X (Jia Qu)