视神经损伤
-
Figure 2|Identification of ADSCs and ADSC-EVs.
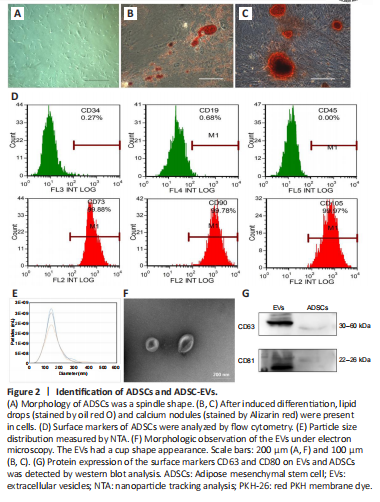
ADSCs are characterized by adherent growth, the positive expression of CD105, CD73, and CD90, the negative expression of CD45, CD34, and CD19, and adipogenic and osteogenic abilities (Dominici et al., 2006). We used primary ADSCs from human adipose tissue, and found that 3rd generation ADSCs presented a spindle-shaped morphology (Figure 2A). More than 95% of cells expressed the positive markers (CD73, CD105, and CD90), and less than 5% of cells expressed the negative markers (CD34, CD45, and CD19) (Figure 2D). The cells had adipogenic and osteogenic abilities (Figure 2B and C). In addition, EVs were enriched from the medium supernatant of ADSCs. These EVs had a cup shape, with a size distribution peak at 136.87 ± 4.52 nm, and were positive for surface markers CD81 and CD63 (Figure 2E–G), consistent with the characteristics of exosomes (Phinney and Pittenger, 2017). These results confirmed the successful isolation of ADSCs and their EVs.
Figure 3|ADCS-EVs reduce glutamate-induced morphological and functional damage in rat retina.
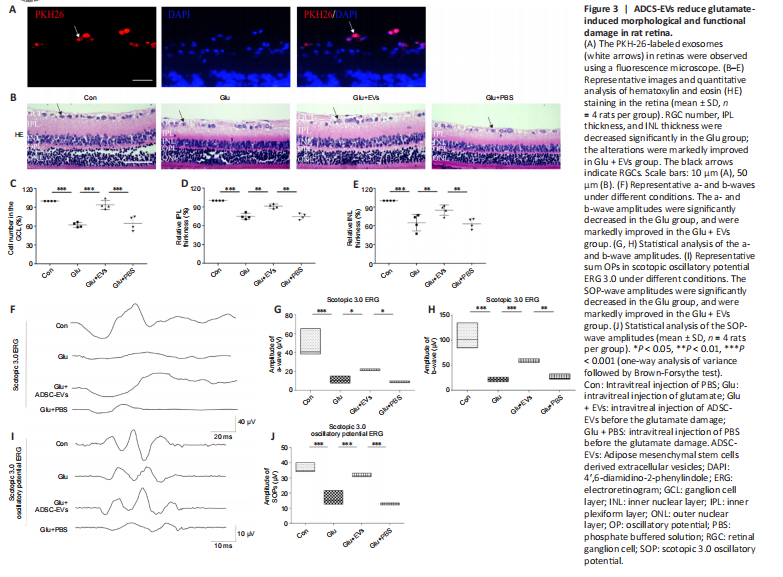
To test the effects of ADSC-EVs on glutamate-induced retinal damage, we injected ADSC-EVs and glutamate into the vitreous sequentially with a 1-hour interval. We found that PKH-26-labeled ADSC-EVs were primarily distributed in RGCs (Figure 3A). Compared with those in the Con group, the number of RGCs (P < 0.001; Figure 3B and C), inner plexiform layer thickness (P < 0.001, Figure 3B and D), inner nuclear layer thickness (P < 0.001; Figure 3B and E), and the amplitudes of the a- (P < 0.001), b- (P < 0.001), and SOP-waves (P < 0.001; Figure 3F–J) in ERG were decreased significantly in the Glu group; these alterations were markedly ameliorated in the Glu + EVs group. These findings suggest that ADSC-EVs decreased glutamate-induced inner retinal damage.
Figure 4|ADSC-EVs reduce intracellular calcium concentration by regulating GluA2 expression in vitro.
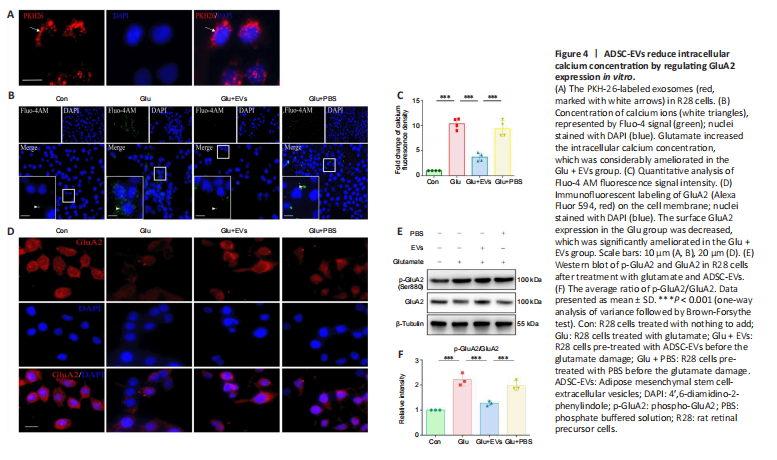
Increased intracellular calcium concentration plays a vital role in glutamate damage (Hartwick et al., 2008). To assess the effects of ADSC-EVs on the elevated intracellular calcium concentration, R28 cells were treated with EVs for 1 hour before the glutamate supplement. PKH-26-labeled ADSC-EVs were distributed mostly in the cytoplasm of R28 cells (Figure 4A). After application of 34 mM glutamate for 8 hours, more than 50% of R28 cells were necrotic (P < 0.001; Additional Figure 1A and B) and intracellular calcium concentration was increased (P < 0.001) compared with control group; these changes were considerably ameliorated in the Glu + EVs group (Additional Figure 1C–F and Figure 4B and C).
Additionally, we observed the effect of ADSC-EVs on AMPAR subunit GluA2 expression and phosphorylation in the vitro model of glutamate damage. Compared with the Con group, glutamate damage led to decreased surface GluA2 expression (Figure 4D) and increased GluA2 phosphorylation in R28 cells (P < 0.001; Figure 4E and F); the changes were significantly ameliorated in ADSC-EV-treated R28 cells (P < 0.001; Figure 4D–F) compared with Glu group. The results suggested that ADSC-EVs reduced the intracellular calcium concentration, upregulated GluA2 expression, and inhibited GluA2 phosphorylation in the in vitro glutamate damage model.