NRR:中南大学黄菊芳团队发现脂肪间充质干细胞来源细胞外囊泡对视网膜兴奋性神经毒性损伤的保护机制
作者:段天婍
兴奋性神经毒性在青光眼、糖尿病视网膜病变和视网膜缺血等多种视网膜疾病的发生发展中都扮演着重要的角色[1, 2]。目前仍缺乏针对视网膜疾病兴奋性神经损伤有效的治疗方案。有研究发现,抑制视网膜兴奋性神经毒性对视力恢复具有重要意义[3]。脂肪间充质干细胞来源于胚胎发育时期的中胚层,是一类具有自我更新和多分化潜能的成体干细胞。其主要从脂肪组织中获取,含量丰富,且取材方便,有利于在未来临床和科研中广泛使用。脂肪间充质干细胞旁分泌的细胞外囊泡可作为其主要产物,具有脂肪间充质干细胞功能的同时,还拥有非免疫原性、低概率异常生长和易到达目标细胞的独特优势[4]。既往研究已经发现,脂肪间充质干细胞对谷氨酸诱导的兴奋性神经毒性损伤具有保护作用[5, 6],但具体机制并不清楚。探索脂肪间充质干细胞来源细胞外囊泡减轻视网膜兴奋性神经毒性的具体机制有助于了解脂肪间充质干细胞来源细胞外囊泡的生物学功能,便于未来利用生物工程技术更加精准和安全地将其用于临床治疗。
最近,中国中南大学黄菊芳团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Adipose mesenchymal stem cell-derived extracellular vesicles reduce glutamate-induced excitotoxicity in the retina”的研究。结果发现,脂肪间充质干细胞来源的细胞外囊泡可明显抑制谷氨酸诱导的视网膜兴奋性神经毒性,并能抑制α-氨基-3-羟基-5-甲基-4-异恶唑丙酸受体(AMPA受体)的钙离子通透性。进一步研究证实,脂肪间充质干细胞来源细胞外囊泡通过抑制蛋白激酶C-α激活从而降低AMPA受体A2亚型(GluA2)磷酸化水平,这一过程稳定了细胞膜上GluA2的表达,从而达到抑制AMPA受体钙离子通透性的作用。这一结果揭示,脂肪间充质干细胞来源的细胞外囊泡作为一种携带脂肪间充质干细胞生物学信息的无细胞制剂,可能在未来视网膜兴奋性神经毒性相关疾病的治疗中起到关键作用。
既往研究发现,谷氨酸诱导的视网膜损伤从形态上主要表现为内层视网膜结构变薄以及视网膜神经节细胞减少[7, 8]。此次研究发现,脂肪间充质干细胞来源的细胞外囊泡能快速进入视网膜,并缓解谷氨酸诱导的视网膜内层组织形态和细胞功能异常(图1)。这说明脂肪间充质干细胞来源的细胞外囊泡具有缓解视网膜兴奋性神经毒性的作用。有研究认为,AMPA受体中GluA2亚型的存在是其阻挡钙离子进入细胞内的关键因素,且GluA2磷酸化可使其在细胞膜上的表达下降[9]。此次研究发现,脂肪间充质干细胞来源的细胞外囊泡能抑制GluA2磷酸化,并稳定细胞膜上GluA2的表达,使细胞内钙离子浓度较谷氨酸损伤时明显下降(图2)。有研究者指出,PKC通路可能是调节GluA2磷酸化的关键因素[10, 11]。黄菊芳等利用蛋白激酶C-α激动剂(12-O-Tetradecanoylphorbol 13-acetate,TPA)刺激GluA2磷酸化,发现脂肪间充质干细胞来源细胞外囊泡的保护作用被抑制(图3)。上述结果说明,脂肪间充质干细胞来源细胞外囊泡能缓解谷氨酸诱导的视网膜兴奋性神经毒性损伤,且其作用与抑制蛋白激酶C-α激活有关。

图1脂肪间充质干细胞来源细胞外囊泡可减轻谷氨酸诱导的大鼠视网膜内层组织形态和细胞功能异常(图源:Duan et al., Neural Regen Res, 2023)
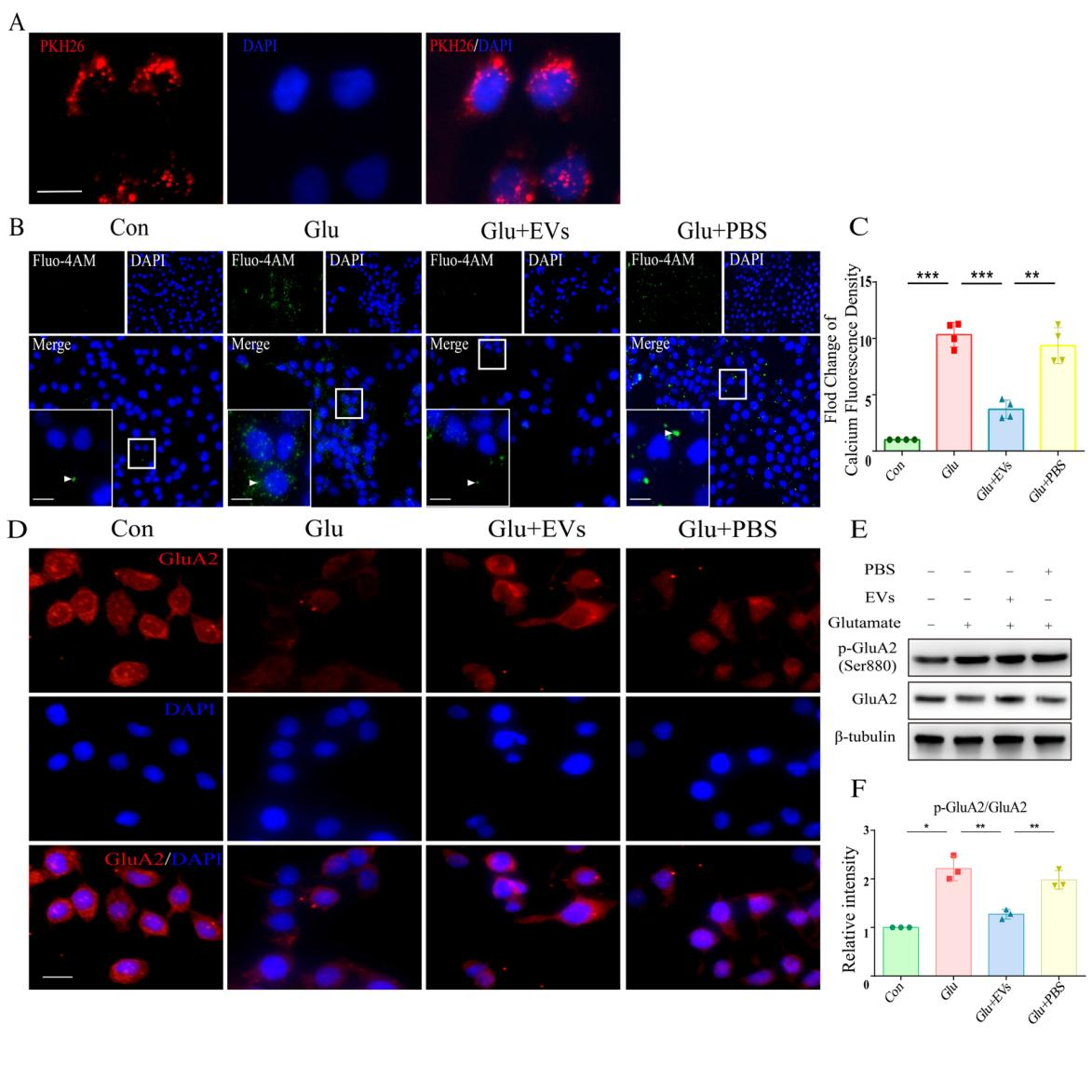
图2脂肪间充质干细胞来源细胞外囊泡可降低谷氨酸损伤细胞中钙离子浓度和GluA2磷酸化水平(图源:Duan et al., Neural Regen Res, 2023)

图3激活蛋白激酶C-α后,脂肪间充质干细胞来源细胞外囊泡对谷氨酸损伤细胞GluA2磷酸化的抑制作用减弱(图源:Duan et al., Neural Regen Res, 2023)
综上所述,通过抑制GluA2和蛋白激酶C-α的磷酸化,脂肪间充质干细胞来源的细胞外囊泡缓解了谷氨酸引起的视网膜损伤。这说明脂肪间充质干细胞来源的细胞外囊泡具有治疗视网膜疾病兴奋性神经毒性损伤的潜力。细胞外囊泡本身就具备了靶向递送药物的潜力,因此,探索其携带物质的作用对将细胞外囊泡应用于眼科疾病治疗具有重要意义。
体内实验中大鼠只观察了在造模后24h,但既往研究认为细胞外囊泡在玻璃体腔内存积并发挥作用的时间远不止24h[12],延长观察时间或许会观察到更显著的保护作用。该研究还未探索细胞外囊泡中调节兴奋性神经毒性的关键成分。
原文链接:https://doi.org/10.4103/1673-5374.369123
参考文献
[1] Alomar SY, B MB, Eldosoky M, et al. Protective effect of metformin on rat diabetic retinopathy involves suppression of toll-like receptor 4/nuclear factor-k B expression and glutamate excitotoxicity. Int Immunopharmacol. 2021;90:107193.
[2] Vernazza S, Oddone F, Tirendi S, et al. Risk factors for retinal ganglion cell distress in glaucoma and neuroprotective potential intervention. Int J Mol Sci. 2021;22(15):7994.
[3] Watanabe K, Asano D, Ushikubo H, et al. Metformin protects against NMDA-induced retinal injury through the MEK/ERK signaling pathway in rats. Int J Mol Sci. 2021;22(9):4439.
[4] Nakano M, Fujimiya M. Potential effects of mesenchymal stem cell derived extracellular vesicles and exosomal miRNAs in neurological disorders. Neural Regen Res. 2021;16(12):2359-2366.
[5] Zhao L, Wei X, Ma Z, et al. Adipose stromal cells-conditional medium protected glutamate-induced CGNs neuronal death by BDNF. Neurosci Lett. 2009;452(3):238-240.
[6] Hao P, Liang Z, Piao H, et al. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab Brain Dis. 2014;29(1):193-205.
[7] Sisk DR, Kuwabara T. Histologic changes in the inner retina of albino rats following intravitreal injection of monosodium L-glutamate. Graefes Arch Clin Exp Ophthalmol. 1985;223(5):250-258.
[8] Zheng S, Yang H, Chen Z, et al. Activation of liver X receptor protects inner retinal damage induced by N-methyl-D-aspartate. Invest Ophthalmol Vis Sci. 2015;56(2):1168-1180.
[9] Cull-Candy SG, Farrant M. Ca(2+) -permeable AMPA receptors and their auxiliary subunits in synaptic plasticity and disease. J Physiol. 2021;599(10):2655-2671.
[10] Purkey AM, Dell'acqua ML. Phosphorylation-dependent regulation of Ca(2+)-permeable AMPA receptors during hippocampal synaptic plasticity. Front Synaptic Neurosci. 2020;12:8.
[11] Guo C, Ma YY. Calcium Permeable-AMPA receptors and excitotoxicity in neurological disorders. Front Neural Circuits. 2021;15:711564.
[12] Mathew B, Ravindran S, Liu X, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. 2019;197:146-160.
 #br#
#br#
通讯作者(左数第七):黄菊芳,研究员,中南大学生命科学学院院长,主要从事神经生物学、人体解剖学与组织胚胎学研究。
第一作者(左数第九):段天婍,在读博士,主要从事眼科学、人体解剖学与组织胚胎学研究。



 #br#
#br#