视神经损伤
-
Figure 1|AAV-mediated transduction of caRheb increases p-S6 levels in RGCs and protects RGCs following ONC by increasing the survival of non-αRGCs.
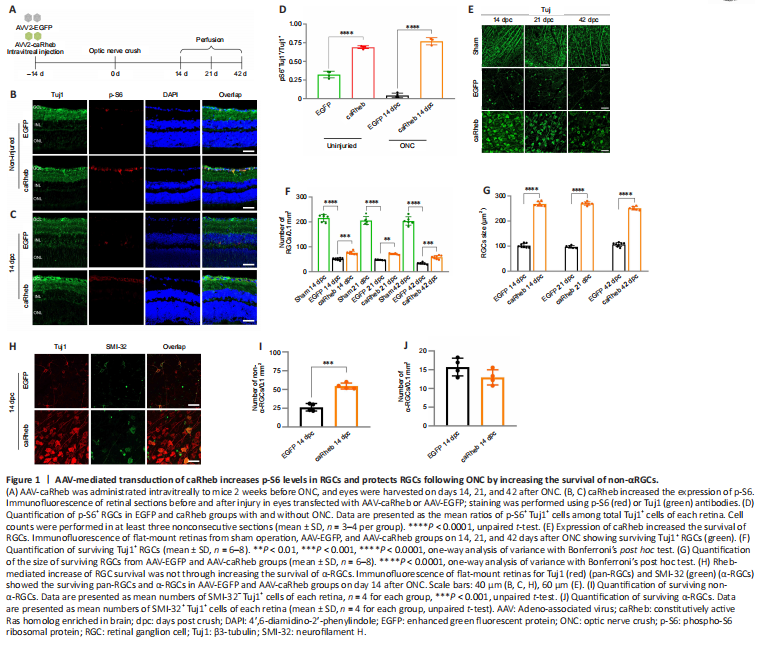
Next, an AAV2 viral vector carrying Rheb(S16H) (AAV-caRheb), a constitutively active form of Rheb (Kim et al., 2011, 2012; Jeon et al., 2015), was injected into the vitreous cavity of mice 14 days before axotomy, and the mice were euthanized on 14, 21, and 42 dpc (Figure 1A). Both AAV-caRheb and AAV-EGFP displayed high transfection efficiency in RGCs (Additional Figure 2), and AAV-EGFP or AAV-caRheb itself did not lead to loss of RGCs (Additional Figure 3A and B). Following the transfection of AAV-caRheb, the percentage of p-S6+ RGCs markedly increased from ~30% to ~70% and remained at a high level even after axotomy (Figure 1B–D), indicative of the constitutive activation of the mTOR pathway mediated by caRheb. Overexpression of caRheb also led to a marked increase in the size of RGCs and exerted neural protective effects after axotomy. RGC cell counts were similar in sham operation groups at all time points (Figure 1E and F). During the post-acute phase of ONC (day 14 post-crush), AAV-caRheb treated mice had higher numbers of RGCs (75.9 ± 6.5/0.1 mm2) compared with AAV-EGFP-treated mice (51.2 ± 4.4/0.1 mm2) (P = 0.0003; Figure 1E and F). There was still a significant protective effect in chronic stages (days 21 and 42 post-crush), in which the caRheb group had significantly more RGCs (21 dpc: 71.2 ± 2.9/0.1 mm2, P = 0.0074; 42 dpc: 58.2 ± 6.7/0.1 mm2, P = 0.0008) than the EGFP group (21 dpc: (47.6 ± 1.9)/0.1 mm2; 42 dpc: 33.6 ± 4.1/0.1 mm2; Figure 1E and F). Moreover, RGC size was significantly larger after caRheb overexpression (Figure 1G).
There are over 30 subtypes of RGCs, and these subtypes can be distinguished by size, morphology, and function. The subtypes differ widely in their susceptibility to damage (V?lgyi et al., 2009; Sanes and Masland, 2015; Daniel et al., 2018). Alpha-RGCs (α-RGCs), which have the largest somas, selectively exhibit high levels of mTOR activity (Duan et al., 2015; Kole et al., 2020) and have been reported as a type of RGC resistant to axotomy (Duan et al., 2015). We used Tuj1 to label pan-RGCs and SMI-32 for α-RGCs (Figure 1H); non-α-RGCs were determined as SMI-32– Tuj1+ cells. The number of non-α-RGCs was increased in the caRheb group compared with the EGFP group (54.6 ± 4.0/0.1 mm2 and 26.3 ± 5.0/0.1 mm2, respectively; P = 0.0001; Figure 1I); however, no significant difference was observed in α-RGCs (Figure 1J). These results indicated that stimulating mTOR activity prefers to rescue those RGC subtypes with low levels of endogenous mTOR activity.
Figure 3|Administration of rapamycin decreases p-S6 levels in RGCs and blocks axon regeneration induced by caRheb.
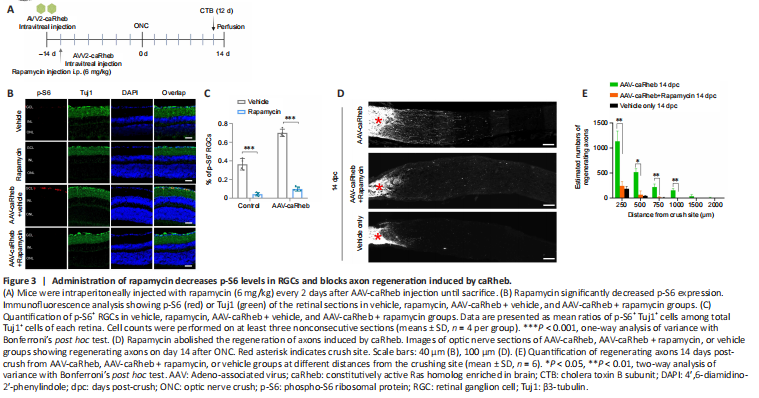
To ascertain the role of mTORC1 in axonal regeneration induced by caRheb, we examined the effects of rapamycin, a specific inhibitor of mTORC1 (Zhou et al., 2009), on regenerating axons. Rapamycin or vehicle was injected intraperitoneally after AAV-caRheb injection (Figure 3A). We found that rapamycin treatment markedly decreased the basal level of p-S6 and attenuated the caRheb transfection-mediated increase in p-S6 (Figure 3B and C). Next, we performed ONC in rapamycin-treated mice 14 days after AAV-caRheb injection. We found that caRheb-mediated axon regeneration was abolished in rapamycin-treated animals (Figure 3D and E), indicating that activation of mTORC1 is necessary for caRheb-induced axon regeneration. Administration of rapamycin in caRheb-overexpressing mice significantly decreased RGC survival (caRheb: 75.9 ± 6.5/0.1 mm2; caRheb + Rapamycin: 66.4 ± 8.1/0.1 mm2, P = 0.0499), but not to levels of the vehicle-only group (Vehicle only: 50.7 ± 2.7/0.1 mm2, P = 0.0019; Additional Figure 4A and B).
Figure 5|S6K1 activation and inhibition of 4E-BP1 are necessary for caRheb-induced axon regeneration following ONC.
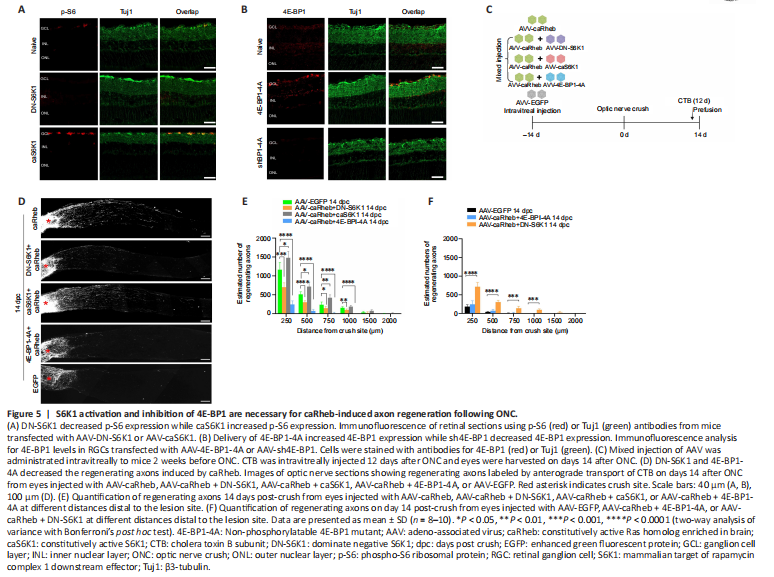
We next investigated the role of S6K1 and 4E-BP1 in caRheb-induced RGC axon regeneration. S6K1 shows a dual potential in axon regeneration (Yaniv et al., 2012; Yang et al., 2014; Al-Ali et al., 2017; Na et al., 2017); we thus constructed an AAV2 vector carrying a dominant-negative mutant of S6K1 (DN-S6K1) and AAV carrying a constitutively active mutant of S6K1 (caS6K1). We also generated an AAV2 viral vector carrying 4E-BP1-4A (a non-phosphorylatable 4E-BP1 mutant (Woodcock et al., 2019)) to overexpress a constitutively active form of 4E-BP1 in RGCs. This mutant is mTORC1-insensitive and constitutively binds to eIF4E to inhibit translation (Thoreen et al., 2012). Approximately 90% of RGCs in retina were infected after AAV2 virus intravitreal injection as determined by Tuj1/HA double labeling (Additional Figure 5). As expected, AAV2-mediated expression of caS6K1 led to a marked increase in p-S6 levels in RGCs, whereas DN-S6K elicited the opposite effect (Figure 5A). Overexpression of 4E-BP1-4A significantly increased 4E-BP1 levels in RGCs (Figure 5B). Equal titers (approximately 2.0 × 1012 genome copies) of AAV-caRheb and AAV-DN-S6K1, AAV-caS6K1, or AAV-4E-BP1-4A were premixed and intravitreally injected 2 weeks before ONC, and CTB was injected to anterogradely label the regenerating axons 2 days before euthanasia (Figure 5C).
Compared with the caRheb alone group, the caRheb + DN-S6K1 group showed significantly decreased numbers of regenerating axons (Figure 5D and E). Considerably more regenerating axons were seen in the caRheb + DN-S6K1 group than in the EGFP (control) group (Figure 5F). These results suggested that phosphorylated S6K1 is a necessary downstream effector of caRheb-induced regeneration. In addition, we found a slight increase in the number of regenerating axons in the caRheb + caS6K1 group compared with the caRheb alone group (Figure 5D). This observation suggested that S6K1 had a positive effect on regeneration in this context.
4E-BP1 is another potential downstream effector of mTORC1 involved in axon elongation (Li et al., 2008; Morita and Sobue, 2009). Compared with the caRheb alone group, the caRheb + 4E-BP1-4A group showed a striking reduction in the numbers of regenerating axons with completely abrogated axon outgrowth (Figure 5D–F). These results suggested that phosphorylation (and inhibition) of 4E-BP1 is necessary for caRheb-induced axon regeneration.
Figure 7|The survival of RGCs after ONC requires the activity of S6K1 and 4E-BP1.
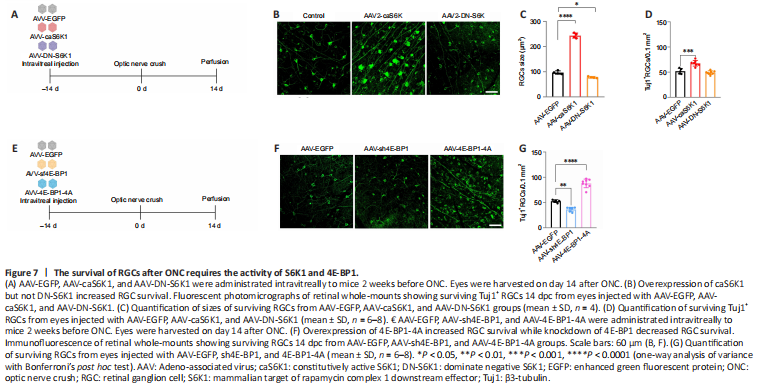
To investigate the role of S6K1 in RGC survival, we performed ONC in mice transfected with the S6K1 mutants and harvested the eyes 14 days post-crush (Figure 7A and B). caS6K1 expression resulted in a signifcantly increased size of RGCs (caS6K1: 243.7 ± 10.1 μm2; EGFP: 96.8 ± 6.7 μm2; P < 0.0001) while DN-S6K1 led to a slight reduction in the size of the cells (DN-S6K1: 77.8 ± 3.1 μm2; P = 0.0139) (Figure 7B and C). Quantification of the remaining RGCs by Tuj1 immunostaining in whole-mount retinas showed that the number of surviving RGCs was higher in the AAV-caS6K1 group (67.2 ± 2.6/0.1 mm2) than in the EGFP group (52.0 ± 5.4/0.1 mm2; P = 0.0001). No difference was observed in the number of surviving RGCs between the DN-S6K1 group (49.1 ± 3.6/0.1 mm2) and the EGFP group after ONC (P = 0.9166; Figure 7B and D).
As we found that 4E-BP1 phosphorylation plays a critical role in axon regeneration, we expected a similar effect of 4E-BP1 on RGC survival. To clarify the critical role of 4E-BP1 incapacitation in RGC survival, we performed AAV-mediated 4E-BP1 knockdown in RGCs (Figure 7E) and found a decreased number of surviving RGCs in the AAV-sh4E-BP1 group (36.3 ± 4.4/0.1 mm2) compared with the AAV-EGFP group (52.7 ± 2.8/0.1 mm2; P = 0.0017). Additionally, 4E-BP1-4A overexpression in ONC retina further increased surviving RGCs number compared with RGCs in the EGFP group (87.2 ± 8.6/0.1 mm2 vs. 52.7 ± 2.8/0.1 mm2, P < 0.0001) (Figure 7F and G). These results suggest that mTORC1-mediated 4E-BP1 incapacitation plays a deleterious role in the survival of RGCs.
Figure 8|Complementary modulation of Rheb and 4E-BP1 strikingly boosts RGC survival.
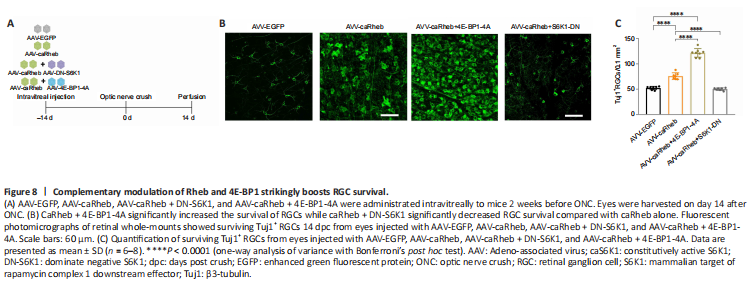
Given these findings, we considered that 4E-BP1 overexpression may exert a protective effect through a different pathway independent of Rheb/mTOR. We therefore co-injected AAV-caRheb and AAV-4E-BP1-4A and tested whether co-expressed caRheb and 4E-BP1-4A had a greater effect on RGC survival than caRheb alone (Figure 8A). The results indeed showed that the co-expression of caRheb and 4E-BP1-4A exerted a more robust protective effect on RGC survival compared with administration of caRheb alone (Figure 8B). The number of surviving RGCs was significantly higher in the caRheb + 4E-BP1-4A group (121.7 ± 8.4/0.1 mm2) than in the EGFP (52.3 ± 3.4/0.1 mm2) and caRheb (75.9 ± 6.5/0.1 mm2) groups (Figure 8C). Co-injection of AAV-DN-S6K1 and AAV-caRheb significantly decreased the number of surviving RGCs compared with AAV-caRheb administration alone (50.2 ± 2.5/0.1 mm2 vs. 75.9 ± 6.5/0.1 mm2, P < 0.0001) (Figure 8B and C), which further confirmed that S6K1 is one of the necessary effectors in caRheb-mediated induction of RGC survival.