神经退行性病
-
Figure 8|The basic structure of dendrimers and their ability to penetrate the blood-brain barrier.
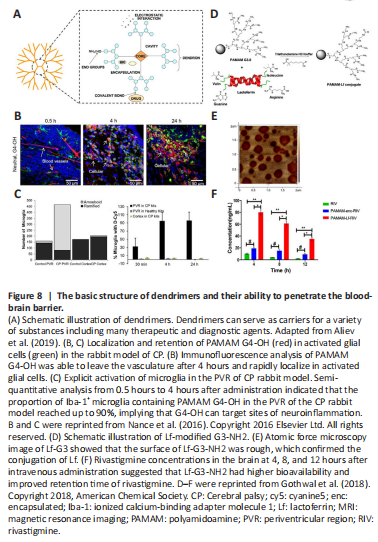
Dendrimers are polymers with highly branched structures and tunable peripheral functional groups. The hydrophobic internal space and the abundance of functional groups provide dendrimers with unique functions, such as the binding sites for drugs, nucleic acids, proteins, or functionalized ligands (Figure 8A). Dendrimers are mostly spherical or disc-like structures, and their morphology is influenced by the size and surface functional groups, which increase with each growing generation (Fana et al., 2020). In the case of PAMAM dendrimers, the number of functional groups on the surface of the polymer increases 2-fold with each generation from 4 in the 0th generation to 64 in the 4th generation, while the shape evolves from relatively asymmetric at the beginning to highly spherical in the 5th and 7th generations (Fana et al., 2020). Moreover, PAMAM and poly(propylene imine) polymers have been found to bind and inhibit the aggregation of Aβ on their own (Aliev et al., 2019). Hydroxy-capped PAMAM dendrimers (G4-OH) more easily penetrated the impaired BBB and targeted the neuroinflammatory microenvironment (Figure 8B and C). The vast majority of G4-OH was present in the activated glial zone in the periventricular region of the cerebral palsy rabbit model at 4 hours after intravenous administration, whereas the G3.5-COOH was not detected until 24 hours (Nance et al., 2016). On this basis, coupling N-acetyl-L-cysteine to a portion of the terminal functional groups of G4-OH (D-NAC) via disulfide bonds significantly enhanced the efficacy of N-acetyl-L-cysteine in inhibiting pro-inflammatory microglia in the brains of rabbits with cerebral palsy. D-NAC resulted in a 3.5-fold reduction in NF-κB expression compared to an equivalent dose of free drug and a more pronounced improvement in the motor function of subjects (Kannan et al., 2012).
The PAMAM dendrimers with targeted modifications also exhibited a greater ability to penetrate the BBB. Lactoferrin-modified G3-NH2 loaded with rivastigmine showed 8- and 4.2-time higher concentrations in the rat brain at 4 hours after administration than the free drug and unmodified carriers, respectively (Figure 8D–F). The overall mobility and memory of rats were enhanced (Gothwal et al., 2018). In addition to PAMAM, one study prepared low-generation (G0 and G1) lysine dendrimers by solid-phase peptide synthesis and successfully incorporated flurbiprofen during the synthesis to form G0K-FP and G1K-FP (Al-Azzawi et al., 2018). Compared to free flurbiprofen, G0K-FP and G1K-FP increased penetration by 12–14% in an in vitro BBB model (Al-Azzawi et al., 2018). Further, lysine dendrimers modified with ApoA-I (the major component of high-density lipoprotein targeting scavenger receptors on the BBB) and NL4 (the peptide targeting neurons) could effectively penetrate the BBB model and deliver BACE1 siRNA to PC12 neuronal cells, thereby downregulating the expression of BACE1 (Zhang et al., 2017a). In a recent study, a multi-targeted dendrimer APBP, which consisted of an 8-arm hydroxylated PEG linked to the ROS-sensitive alkyne group followed by binding to phosphorylated nuclear factor E2-related factor 2 (Nrf2) (activating the Nrf2-mediated signaling pathway) with modification by Aβ peptide (RAGE ligand), could target the inflammatory microenvironment of AD effectively to reduce neuroinflammation and ROS after penetrating the BBB and modulating the activated phenotype of microglia, thereby reducing Aβ burden and improving cognitive function in an AD mouse model (Liu et al., 2021b). Dendrimers are a very promising category of brain-targeting drug delivery vehicles owing to their high capacity of drug loading and amenability of modification; however, special attention should be paid to their possible cytotoxicity with an increasing number of branches.
Figure 9|Preparation of nanogels and prospects for the treatment of Alzheimer’s disease.
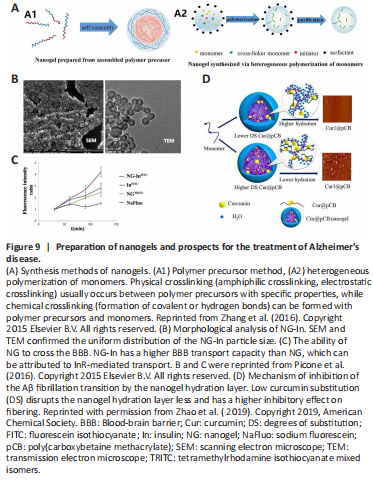
Nanogels (NGs) are defined as nanoscale hydrogels, that are three-dimensional polymer networks formed by physical or chemical cross-linking and have the characteristics of both hydrogels and NPs (Figure 9A; Zhang et al., 2016). Swelling is the most important property of NGs and can be triggered by physicochemical changes such as temperature, pressure, pH, ions, and specific molecular recognition. Nanogels are gaining increasing attention as drug-delivery carriers owing to their excellent drug-loading capacity, high stability, biocompatibility, and controlled release of drugs through controlled swelling (Zhang et al., 2016). One study prepared poly(N-vinylpyrrolidone)-co-acrylic acid NG by the ionizing radiation method and then coupled it with insulin to obtain NG-In. In vitro experiments showed that the NG-In was more efficient than free insulin in penetrating the BBB model (Figure 9B and C), and it activated Akt at almost twice the level of the equivalent quantity of free insulin, completely reversing the Aβ-induced cytotoxicity (Picone et al., 2016). Another study designed angiopep-2-modified CS NGs to deliver oxytocin to the brain to treat AD in the early stage. Angiopep-2-modified CS NGs can be enriched in brain regions with AD-like pathologies and effectively inhibit the activation of microglia and reduce the levels of inflammatory cytokines by blocking NF-κB and MAPK-related signaling pathways (Ye et al., 2022). Regular treatment of APP/PS1 mice with angiopep-2-modified CS NGs starting at age 12 weeks decelerated the progression of Aβ deposition and neuronal apoptosis, and prevented cognitive impairment and delayed hippocampal atrophy (Ye et al., 2022). As drug delivery carriers, the hydration layer of the NGs can inhibit the conformational transition to β-sheet in Aβ and thus reduce its neurotoxicity. However, some loaded drugs can disrupt the hydration layer (Figure 9D; Zhao et al., 2019). Therefore, for better synergy between NGs and loaded drugs to produce better outcomes for the treatment of AD, it is required to pay attention to adequately protect the hydration layer or enhance hydration in designing the NG formula.
点击此处查看全文