神经损伤与修复
-
Figure 2|Preparation and characterization of MXenes.
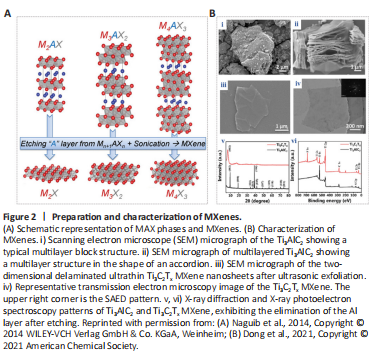
MAX phases are a large family containing more than 50 members consisting of transition metal carbides, nitrides, or carbon nitrides (Naguib et al., 2011; Anasori, et al., 2017). The “M” is transition metal element, the “A” is a main family element, and the “X” is nitrogen or carbon (Figure 2A; Naguib et al., 2014). The basic formula can be expressed as M(n+1)AXn. Moreover, in the perspective of biomedical engineering, the most popular materials in this family are Ti3SiC2, Ti3C2Tx, and the multilayer Ti3C2 (Huang et al., 2022). MXenes are typically prepared using a top-down stripping method with selective stripping of the A element layer from the precursors to convert to an MAX phase or non-MAX phase (Naguib et al., 2014).
To produce monolayer MXenes, simple mechanical peeling is not efficient enough, and intercalators need to be used. Delamination intercalators mainly include metal ion intercalators (such as metal hydroxides or halide salts) and organic intercalators (such as TBAOH) (Alhabeb, et al., 2017; Yu et al., 2017). Dong et al. (2021) reported the procedure of converting multilayer Ti3AlC2 into the layered Ti3C2Tx MXene. MAX phases have a typical multilayer block structure, which is similar to an accordion structure, after selective etching and 2D nearly transparent ultrathin delaminates after ultrasonic exfoliation (Figure 2B; Dong et al., 2021). There are a few bottom-up synthesis methods of MXenes. Xu et al. (2015) synthesized ultra-thin α-Mo2C 2D crystals with a transverse size of up to 100 mm on Cu/Mo foil using chemical vapor deposition. They made W and Ta into ultra-thin WC and TaC crystals. The MXenes prepared using their method have a large lateral size. Compared with top-down manufacturing methods, the bottom-up synthesis methods usually start from small organic or inorganic molecules/atoms (Huang et al., 2018). They also have the advantages of manipulating the size distribution and morphology of MXenes, but the surface termination is less controllable. This may be due to the complex composition of MXenes.
Figure 3|Biocompatibility of MXene films for neuronal growth .
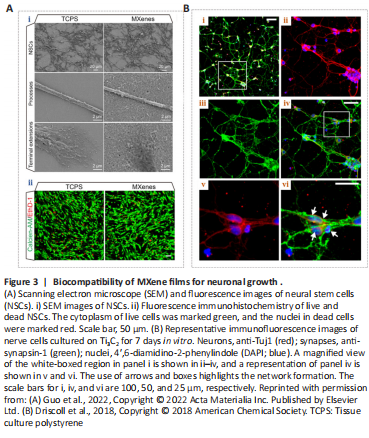
Figure 4| Effects of MXenes on neural stem cell differentiation.
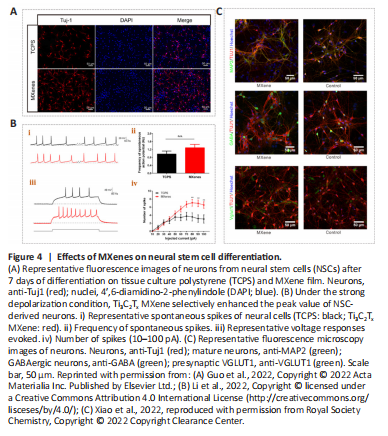
MXenes have already shown great promise in biomedical applications, although their biocompatibility and potential toxicity must be taken into consideration. The biocompatibility of MXenes has been reported in a variety of cell types. Guo et al. (2022) dispersed the Ti3C2Tx MXene with abundant surface functional groups on tissue culture polystyrene (TCPS) and cultured neural stem cells (NSCs) on the material after coating it with laminin to investigate regulatory effects on the cell survival and behavior. The researchers found that these cells were cultured on both the Ti3C2Tx MXene and TCPS forms with stable adhesion, additionally exhibiting extensive spreading of their terminal extensions (Figure 3Ai). Histology of living and dead NSCs (Figure 3Aii) showed that Ti3C2Tx MXenes had good biocompatibility and were an excellent neural interface material. Although the NSCs on these two materials had similar proliferative abilities, the Ti3C2Tx MXene film showed more efficient neuronal differentiation (Figure 4A). Moreover, compared with TCPS substrates, cells on Ti3C2Tx MXenes had longer neurites, more branches and tips, and were more active, mature, and more highly differentiated, implying that Ti3C2Tx MXene was an efficient interface for NSC-derived neurons.
Driscoll et al. (2018) performed nerve recordings in vivo using Ti3C2 MXenes and reported good biocompatibility with immortalized tumor cell lines and the rat brain. They investigated the cytotoxicity of Ti3C2 films on primary cortical neurons, and immunocytochemistry results exhibited a widespread network on both substrates (Figure 3B; Driscoll et al., 2018). Their results demonstrated that the primary cortical neurons could adhere to, grow on, and form neuronal networks on Ti3C2 and TCPS. This material provided a high-resolution neural interface for neuroelectronic devices. This research can greatly broaden the potential applications of the MXene families for neuroscientific research. Zhang et al. (2019a) implanted Ti3C2Tx MXenes into Sparague-Dawley rats and found that MXenes were actively taken up via cell-mediated phagocytosis. In another study, a Ti3C2Tx MXene film showed no cytotoxicity to NSCs (Zhang et al., 2022), and no cytotoxicity was detected in nerve tissue (Vural et al., 2020; Wu et al., 2020). The above studies show that MXenes are biocompatible and are capable of supporting neuron growth and network formation (Wang et al., 2021; Guo et al., 2022).
Li et al. (2022) reported that ES coupled with Ti3C2Tx MXene could ensure normal growth of NSCs and markedly enhance proliferation in NSCs, which also exhibited higher neuronal differentiation efficiency, implying that Ti3C2Tx MXenes can promote the maturation of NSCs. Ion channels are known to be essential for the fate and function of nerve cells (Yin et al., 2019). Li et al. (2022) investigated effects of MXenes on ion channels, synaptic connections between cells, and neural network formation in newborn neurons using patch clamp electrophysiology (Figure 4B). There was no significant difference in voltage-gated Na+/K+ currents when cells were in close contact with Ti3C2Tx MXenes, but the amplitude of the voltage-gated Ca2+ current was selectively increased, which may account for longer neurites. Further, Ti3C2Tx MXene promoted neuronal differentiation during strong depolarizations and increased the frequency of synaptic release, thereby enhancing synaptic transmission. In addition, Xiao et al. (2022) applied an uncoated Ti3C2Tx MXene to explore the development of hippocampal cells. Using electrophysiological recordings, they tested the effects of uncoated Ti3C2Tx MXenes on cultured neuronal cells and the activity of neuronal microcircuits; this MXene was suggested to preserve basic physiological functions in neuronal circuit development (Figure 4C). Their work makes it possible to construct neural repair devices that can be applied in research, diagnosis, and therapy.Figure 5| MXene-Matrigel regulates the formation of neural networks in hair cells and spiral ganglion neurons.
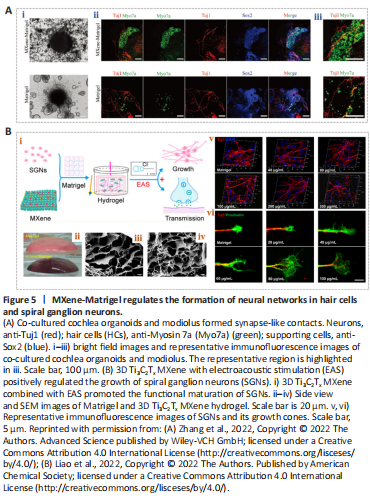
Organoids are mini-organs with various cell types that are derived from stem cells of various tissues and organs, such as embryonic stem cells or induced pluripotent stem cells (Huch and Koo, 2015; Nengzhuang et al., 2022). They have highly similar cellular compositions and physiological characteristics to real organs, providing marked advances in research on nervous system development and disease (Di Lullo and Kriegstein, 2017). Cochlear organoids (Cochlear-Orgs) have been successfully formed in 3D in vitro using induced pluripotent stem cells and embryonic stem cells (Lee et al., 2017; McLean et al., 2017). However, difficulties remain in the simulation of extracellular environments (Gjorevski et al., 2016; Roccio et al., 2018). To better simulate the cellular environment, Zhang et al. (2022) combined Ti3C2Tx MXene nanomaterials with Matrigel to establish a co-culture system between Cochlear-Orgs and spiral ganglion neurons (SGNs) in vitro. The 3D hydrogel containing a certain concentration of Ti3C2Tx MXene was appropriate for Cochlea-Orgs and had the ability to promote the formation, maturation, and proliferation of organoid hair cells (Figure 5A; Zhang et al., 2022). In addition, they demonstrated that this 3D Ti3C2Tx MXene could facilitate the establishment of neural connections between hair cells and SGNs, and it increased the efficiency of synapse formation.
The culture of neural cells remains restricted in our ability to regulate the survival, regeneration, and function of SGNs. Low-frequency bidirectional postoperative electrical stimulations has been found to stimulate axonal regeneration in neural cells and to reduce neuron degeneration (Kopelovich et al., 2013). Cochlear implants can convert sound information into electrical signals, which can be precisely delivered to targeting functional SGNs (Ramsden et al., 2012). This may serve as an effective means of stimulation. Liao et al. (2022) constructed a 3D electroacoustic stimulation system that consists of a cochlear implant device and conductive Ti3C2Tx MXene-Matrigel hydrogel. The 3D Ti3C2Tx MXene hydrogel positively regulated the growth and maturation of SGNs (Figure 5B). In addition, the SGNs exhibited a greater number of synapses and higher calcium oscillation frequencies, suggesting that the 3D Ti3C2Tx MXene system could promote the formation of mature synapses and intercellular signal transmission. This research showed the potential value of MXenes in promoting the regeneration and recovery of the auditory nerve after injury.