神经损伤与修复
-
Figure 1|SNI decreases PWT and lowers GPR39 expression in the spine.
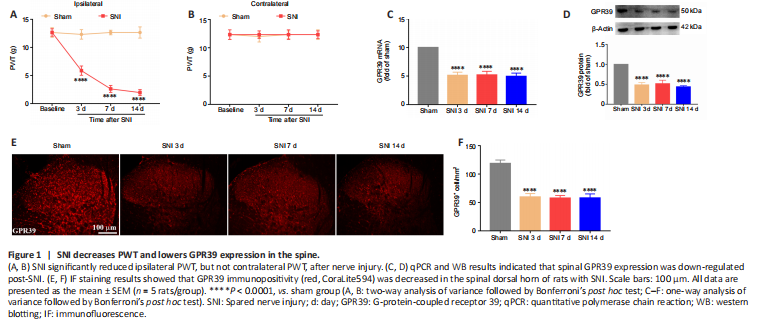
Figure 2|GPR39 localization in the spinal cord.
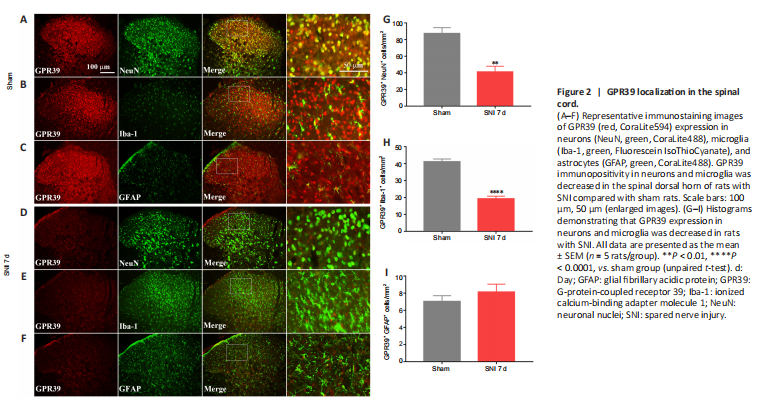
结果:A neuropathic pain model was conducted by SNI in rats. The behavioral results showed that SNI decreased the ipsilateral PWT (Figure 1A), but not the contralateral PWT (Figure 1B). We then assessed GPR39 expression levels in rats with neuropathic pain. The results indicated that GPR39 mRNA (Figure 1C) and protein (Figure 1D) levels were down-regulated following SNI. Furthermore, single immunostaining confirmed that SNI decreased GPR39 immunopositivity in the spinal dorsal horn (Figure 1E and F). Moreover, as shown in Figure 2, double immunostaining indicated that GPR39 mainly co-localized with NeuN and Iba-1, and that SNI reduced GPR39 immunopositivity in neurons and microglia. These results substantiated SNI diminished the expression of GPR39 in the spinal cord of rats.
Figure 3|Spared nerve injury inactivates the SIRT1/PGC-1α pathway and impairs mitochondrial biogenesis.
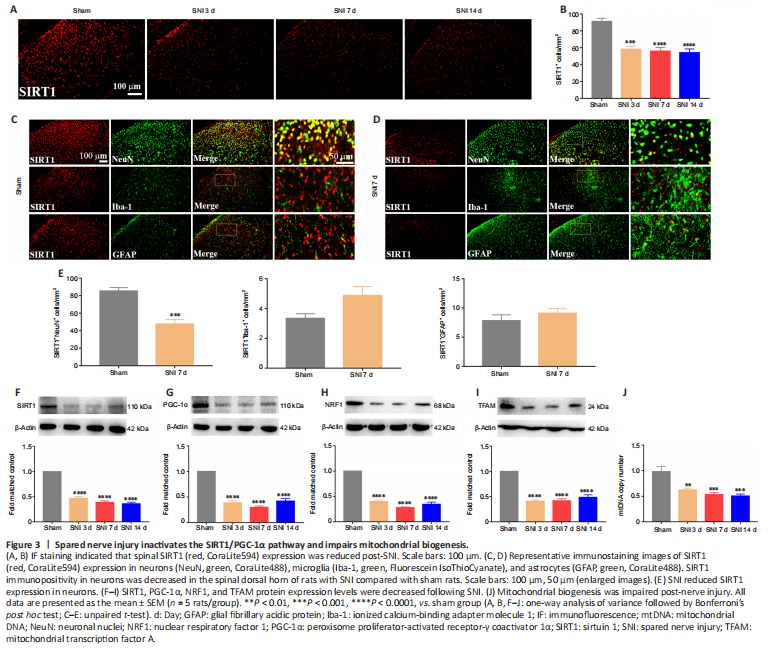
结果:It was demonstrated that activation SIRT1/PGC-1α promoted mitochondrial biogenesis. We then examined the expression levels of the mitochondrial biogenesis pathway-related proteins SIRT1, PGC-1α, and NRF1/TFAM after SNI. The immunostaining results indicated that SIRT1 expression was down-regulated in the spinal dorsal horn of the neuropathic pain group compared with the sham group (Figure 3A and B). Moreover, double immunostaining demonstrated that SNI decreased the SIRT1 expression in neurons (Figure 3C–E). Furthermore, the WB results revealed that SIRT1 (Figure 3F), PGC-1α (Figure 3G), NRF1 (Figure 3H), and TFAM (Figure 3I) expression were significantly decreased in rats with SNI, compared with sham rats. In addition, neuropathic pain significantly decreased the mtDNA copy number (Figure 3J). The results indicated that SNI suppressed SIRT1/PGC-1α and NRF1/TFAM-mediated mitochondrial biogenesis in the spinal cord of rats.
Figure 5|TC-G 1008 reverses neuropathic pain-induced impairment of mitochondrial biogenesis and neuroinflammation by activating the SIRT1/PGC-1α pathway.
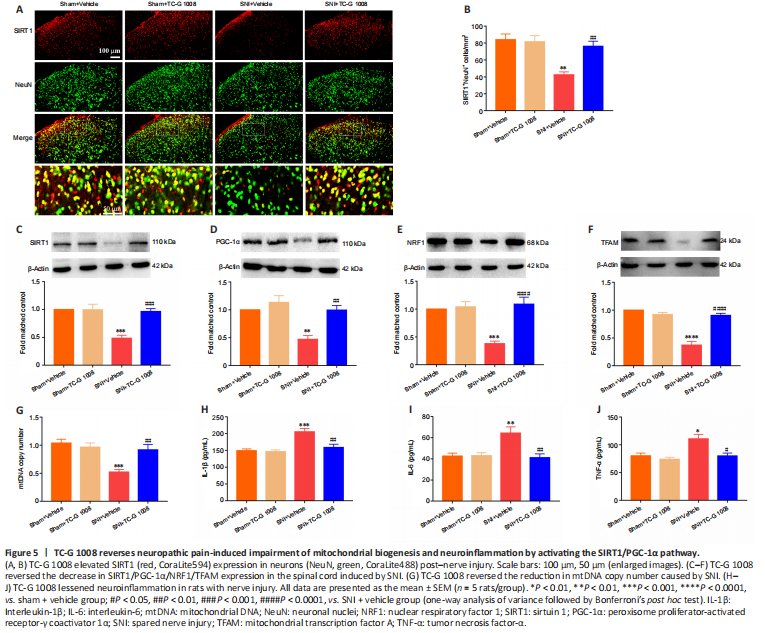
结果:SIRT1/PGC-1α played a crucial role in regulating mitochondrial biogenesis and inflammatory activities, and GPR39 could regulate the down-stream of SIRT1. But it was unclear whether activation of GPR39 could enhance mitochondrial biogenesis and alleviate neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI. Immunofluorescence staining revealed that TC-G 1008 reversed SNI-induced down-regulation of SIRT1 in neurons (Figure 5A and B). In agreement with this, WB showed that administration of TC-G 1008 significantly augmented SIRT1 levels (Figure 5C) and rescued the decrease in PGC-1α (Figure 5D), NRF1 (Figure 5E), and TFAM (Figure 5F) expression caused by SNI. Moreover, TC-G 1008 counteracted the impairment of mitochondrial biogenesis observed in rats with nerve injury (Figure 5G). In addition, TC-G 1008 inhibited the IL-1β (Figure 5H), IL-6 (Figure 5I), and TNF-α (Figure 5J) secretion induced by neuropathic pain. The data demonstrated that activation of GPR39 promoted NRF1/TFAM-mediated mitochondrial biogenesis and attenuated neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI.
Figure 7|Beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation are abrogated by intrathecal injection with GPR39 siRNA in rats with SNI.
结果:To further determine the role of GPR39 in neuropathic pain, GPR39 siRNA was used in this study. Double immunofluorescence staining showed that the increase in SIRT1 expression in neurons induced by TC-G 1008 was abolished by GPR39 siRNA (Figure 7A and B). Additionally, WB showed the increase in SIRT1 (Figure 7C), PGC-1α (Figure 7D), NRF1 (Figure 7E), and TFAM (Figure 7F) expression levels induced by TC-G 1008 was reversed by injection with GPR39 siRNA in rats with SNI. TC-G 1008 mitigated the impairment of mitochondrial biogenesis, whereas GPR39 siRNA abolished this effect (Figure 7G). However, GPR39 silencing reversed the improved neuroinflammation induced by TC-G 1008 in rats with SNI (Figure 7H–J). The data further demonstrated that activation of GPR39 promoted NRF1/TFAM-mediated mitochondrial biogenesis and attenuated neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI.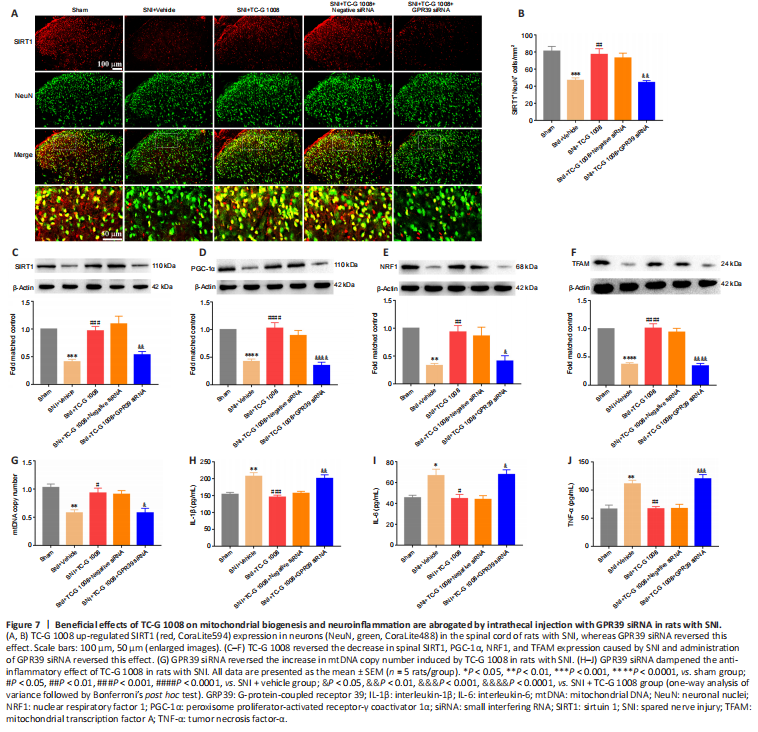
点击此处查看全文