脑损伤
-
Figure 1|TWS119 activates the Wnt/β-catenin pathway, relieves oxidative stress, and rescues downregulated expression of tight junction and CYP1B1 proteins after stroke.
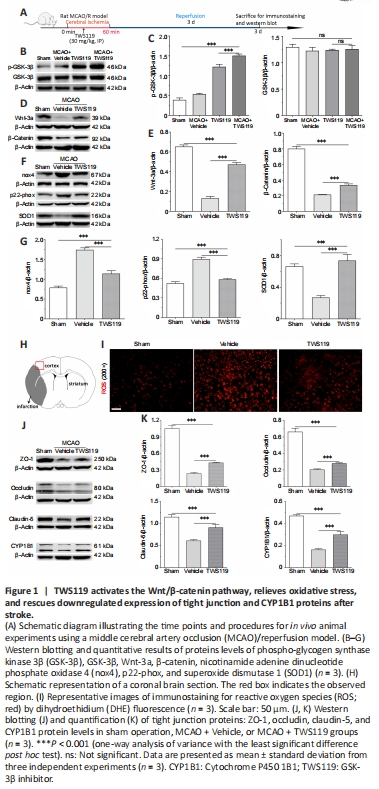
We first investigated the effect of the pharmacological inhibitor, TWS119, on the levels of GSK-3β and its phosphorylated protein under cerebral ischemia conditions. A schematic diagram illustrating the time points and procedure for the rat MCAO/reperfusion experiments are shown in Figure 1A. GSK-3β is inactivated by phosphorylation or inhibited by TWS119. Western blotting showed that protein expression of phospho-GSK-3β (p-GSK-3β) was weakly detectable in the sham operation group, while total GSK-3β protein expression was highly expressed. Interestingly, the level of p-GSK-3β was increased after treatment with TWS119, and further increased in the MCAO plus TWS119 group (P < 0.001; Figure 1B and C). However, there was no significant difference of total GSK-3β protein levels between the sham operation, MCAO + Vehicle, TWS119, and MCAO plus TWS119 groups (P > 0.05; Figure 1B and C). This shows that GSK-3β is inactivated by TWS119 after stroke.
Next, we explored whether administration of TWS119 activates or suppresses the Wnt/β-catenin pathway under stroke conditions. The results indicate that levels of key pathway-related proteins (namely β-catenin and Wnt-3a) were lower in the MCAO + Vehicle group compared with the sham operation group, and higher in the TWS119 group than the MCAO + Vehicle group following stroke (P < 0.001; Figure 1D and E). This suggests that treatment with TWS119 contributes to activation of the Wnt/β-catenin pathway after stroke.
We further determined the levels of cellular oxidative stress-related factors, such as nox4 and p22-phox proteins, which were markedly upregulated and higher in the MCAO + Vehicle group than in the sham operation group; however, TWS119 treatment lowered the levels of these proteins (P < 0.001; Figure 1F and G). Conversely, cerebral ischemia led to downregulation of the antioxidative stress-related factor, superoxide dismutase 1 (SOD1), a trend that was reversed by treatment with TWS119 (P < 0.001; Figure 1F and G). This finding was also supported by ROS data via DHE fluorescence staining. ROS levels were significantly downregulated in ischemic rats treated with TWS119 compared with the MCAO + Vehicle group (Figure 1H and I).
Previous evidence confirmed that TWS119 protected the BBB against ischemia-induced injury in a rat stroke model (Wang et al., 2016). Similar to these findings, we observed that downregulated expression of TJ proteins (namely, zonula occludens-1 [ZO-1], occludin, and claudin-5) were rescued in the TWS119 group compared with the vehicle group (P < 0.001; Figure 1J and K). Immunofluorescence staining showed that mean fluorescence intensity of CD31-positive cells was stronger in the MCAO + TWS119 group than in the MCAO + Vehicle group (P < 0.001); CD31 is a marker of vascular endothelial cells (Additional Figure 1A and B). Interestingly, CYP1B1 protein levels were upregulated after TWS119 treatment in the MCAO + TWS119 group compared with the MCAO + Vehicle group (P < 0.001; Figure 1J and K). Together, the results imply that TWS119 rescues downregulated expression of TJs and CYP1B1 protein, and protects the BBB after stroke.
Figure 2|OGD/R induces oxidative stress and downregulates β-catenin and CYP1B1 proteins in RBMVECs.
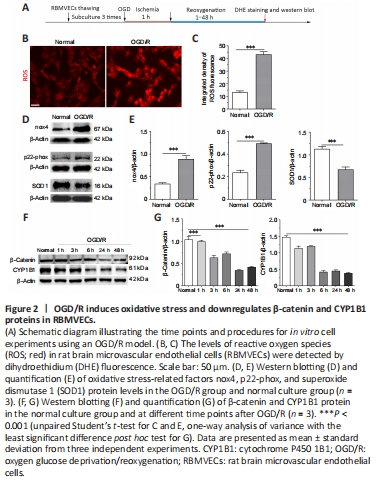
As shown in Figure 2A–C, compared with the normal condition culture group, the levels of ROS detected by DHE fluorescence staining were significantly elevated in the OGD/R group (P < 0.001), showing successful establishment of OS in RBMVECs under OGD/R conditions. Similarly, the levels of nox4 and p22-phox proteins were higher in the OGD/R group compared with the normal culture group, while SOD1 expression was downregulated after OGD/R injury (P < 0.001; Figure 2D and E). Altogether, this indicates that OS is triggered by OGD/R exposure in RBMVECs.
Under OGD/R conditions, β-catenin can translocate from the cytoplasm to the nucleus (Additional Figure 2). However, inhibition of Wnt/β-catenin signaling was detected in RBMVECs, as confirmed by decreasing protein levels of β-catenin. We found that β-catenin protein levels decreased in the OGD/R group along with time of reoxygenation after OGD, reaching their lowest level between 24 to 48 hours after reoxygenation. In addition, a similar trend in CYP1B1 protein levels were observed in cells that underwent OGD/R (Figure 2F and G). In summary, these results suggest that OS suppresses the activity of Wnt/β-catenin signaling and CYP1B1 protein expression.
Figure 3|Effect of β-catenin overexpression on oxidative stress, tight junction proteins, and cell apoptosis in RBMVECs under OGD/R conditions.
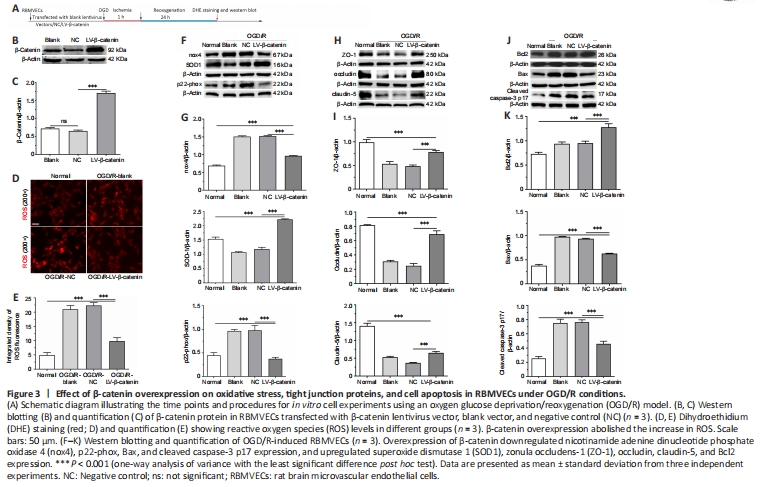
The data described so far, as well as other evidence, confirms that OS inhibits Wnt/β-catenin signaling. Yet whether this signaling alleviates or exacerbates OS remains controversial (Park et al., 2010; Sun et al., 2021). First, compared with the blank vector or NC group, protein expression levels of β-catenin were markedly upregulated in cells transfected with overexpressed β-catenin (i.e., the LV-β-catenin group) (P < 0.001; Figure 3A–C). As expected, enhanced Wnt/β-catenin pathway activation contributed to alleviation of cellular OS. DHE staining showed that the fluorescence intensities of ROS were stronger in the OGD/R groups compared with the normal condition culture group (P < 0.001). However, the LV-β-catenin group exhibited lower levels of ROS compared with the blank group and NC group (P < 0.001; Figure 3D and E).
Similarly, we found that nox4 and p22-phox protein levels were significantly increased in the OGD/R groups compared with the normal condition culture group (P < 0.001). However, this rising trend was partly abolished by overexpression of the β-catenin gene, and protein levels in the LV-β-catenin group were markedly lower compared with the NC group (P < 0.001; Figure 3F and G). In contrast, the antioxidative-related protein, SOD1, showed upregulated levels in the LV-β-catenin group compared with the NC group (P < 0.001; Figure 3F and G). This suggests that activation of the Wnt/β-catenin pathway contributes to alleviation of OS in an in vitro ischemia simulation model in RBMVECs.
In addition, we found that β-catenin overexpression reversed the downregulation of ZO-1, occludin, and claudin-5 proteins in RBMVECs after OGD/R. Levels of these three proteins were significantly upregulated in the LV-β-catenin group compared with the blank and NC groups (P < 0.001; Figure 3H and I).
Furthermore, we observed that β-catenin overexpression ameliorated cell apoptosis after OGD/R. Expression of the antiapoptotic protein, Bcl2, was elevated in the LV-β-catenin group compared with the NC group, whereas the proapoptotic-associated proteins, Bax and cleaved caspase-3 p17, were significantly decreased in the LV-β-catenin group compared with the NC group (P < 0.001; Figure 3J and K). Altogether, the data demonstrate that β-catenin overexpression induced augmentation of the Wnt/β-catenin pathway that may attenuate BBB damage and improve cell survival following exposure to OGD/R conditions.
Figure 4|Effect of β-catenin overexpression on migration ability of rat brain microvascular endothelial cells under oxygen glucose deprivation/reoxygenation conditions.
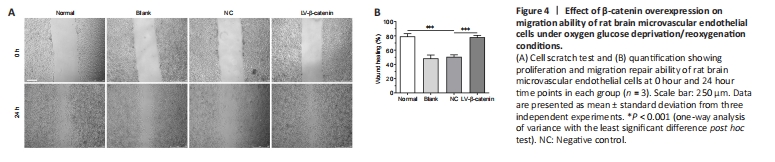
As shown in Figure 4, RBMVECs in the normal condition culture group rapidly migrated to heal a wound 24 hours after scratching. Interestingly, cellular wound healing was significantly decreased after OGD/R. However, β-catenin overexpression improved the proliferation and migration ability of RBMVECs after OGD/R. At the 24 hour time point after OGD/R, compared with the NC group, wound healing percentage of RBMVECs transfected with LV-β-catenin was significantly increased (wound healing percentage: LV-β-catenin group vs. NC group, P < 0.001; Figure 4).