周围神经损伤
-
Figure 1|Runx2 up-regulation in Schwann cells following peripheral nerve injury.
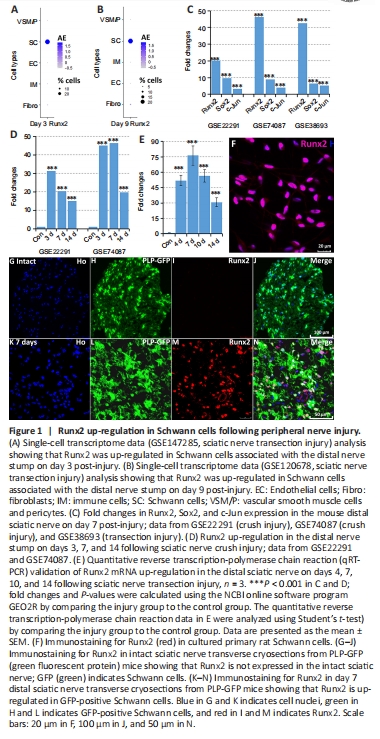
Our research group is interested in understanding the function of transcription factors that are up-regulated in injured peripheral nerves (Doddrell et al., 2012, 2013; Mindos et al., 2017; Roberts et al., 2017; Dun et al., 2019). Recently, we analyzed cell type-specific gene up-regulation by single-cell RNA sequencing data analysis in intact and injured mouse sciatic nerves (Chen et al., 2021) and found that the transcription factor Runx2 was primarily up-regulated in Schwann cells associated with injured peripheral nerves (Figure 1A and B). Here, we analyzed Runx2 expression in the mouse distal nerve stump using three publicly available microarray data sets: GSE22291 (crush injury), GSE74087 (crush injury), and GSE38693 (transection injury) (Barrette et al., 2010; Arthur-Farraj et al., 2012; Pan et al., 2017). This analysis revealed that Runx2 is dramatically up-regulated in the mouse distal sciatic nerve stump following both sciatic nerve crush injury and transection injury (Figure 1C and D). At day 7 post-injury, Runx2 showed a significantly higher fold change (P < 0.001) in up-regulation in the distal sciatic nerve than either c-Jun or Sox2, two well characterized and regulated transcription factors (Parkinson et al., 2008; Parrinello et al., 2010; Arthur-Farraj et al., 2012; Fontana et al., 2012; Roberts et al., 2017; Dun et al., 2019) (Figure 1C). Following the microarray data analysis, we performed qRT-PCR analysis in a mouse (C57/BL6) model of sciatic nerve transection injury and observed dramatic up-regulation of Runx2 mRNA expression up to 14 days post-injury (Figure 1E). We next examined Runx2 protein expression in cultured rat primary Schwann cells and in sciatic nerve sections 7 days after injury in PLP-GFP mice (in which Schwann cells are labeled with GFP) (Mallon et al., 2002; Chen et al., 2019). Immunostaining demonstrated strong Runx2 expression in cultured rat Schwann cells (Figure 1F), and the in vivo staining results confirmed that Runx2 was primarily up-regulated in Schwann cells (GFP-positive) associated with the distal nerve following injury (Figure 1G–N).
Figure 3|Generating Schwann cell-specific Runx2 knockout mice.
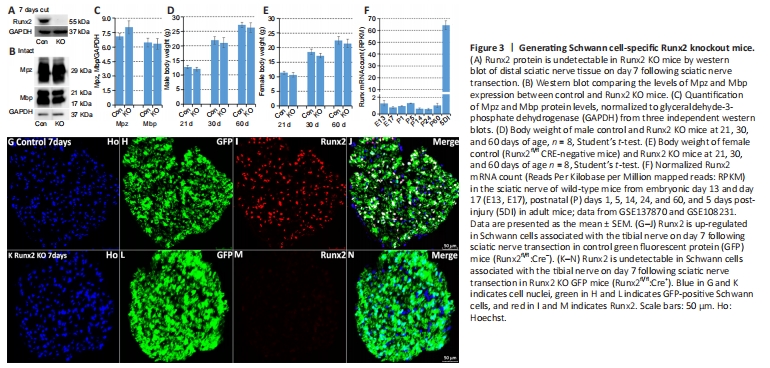
Figure 4|Normal peripheral nerve development in Schwann cell-specific Runx2 knockout mice.
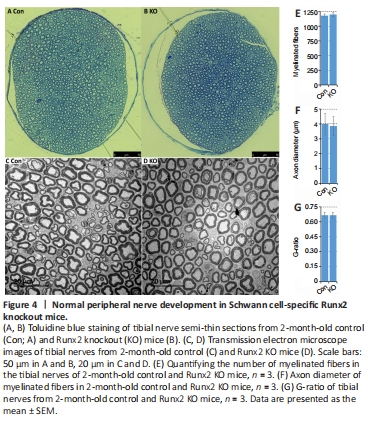
To better understand the role of Runx2 in Schwann cells during peripheral nerve regeneration, we crossed Runx2fl/fl mice (Ferrari et al., 2015) with P0-CRE (mP0-TOTACRE) mice (Feltri et al., 1999) to generate Schwann cell-specific Runx2 KO mice. Complete Runx2 knockout in Schwann cells was confirmed by western blot (Figure 3A) and immunostaining (Figure 3G–N) on day 7 post-sciatic nerve transection. A previous study reported that Runx2 global null mice died soon after birth due to ossification failure (Otto et al., 1997). However, our Schwann cell-specific Runx2 KO mice were viable and exhibited normal body weights compared with control mice (Runx2fl/fl CRE negative) of the same age (Figure 3D and E). Furthermore, western blotting revealed similar levels of Mpz and myelin basic protein (Mbp) in control and Runx2 KO mice (Figure 3B and C). Next, we assessed Runx2 mRNA expression in the developing sciatic nerve of wild-type mice and found that all Runx2 mRNA counts were below 2 reads per kilobase of transcript per million reads mapped (RPKM) from embryonic day 13 to postnatal day 60 (Figure 3F). Following sciatic nerve injury on day 5, the Runx2 mRNA count increased to 64.36 RPKM (Figure 3F), suggesting that Runx2 is expressed at very low levels in the developing mouse sciatic nerve. In agreement with this, examination of semi-thin sections of tibial nerve (Figure 4A and B) and transmission electron microscopy images of tibial nerve sections (Figure 4C and D) showed normal myelination, unaltered axon diameter, and unchanged G-ratios in 2-month-old Runx2 KO mice compared with controls (Figure 4E–G).
Figure 5|Increased Schwann cell proliferation in Runx2 knockout (KO) mice.
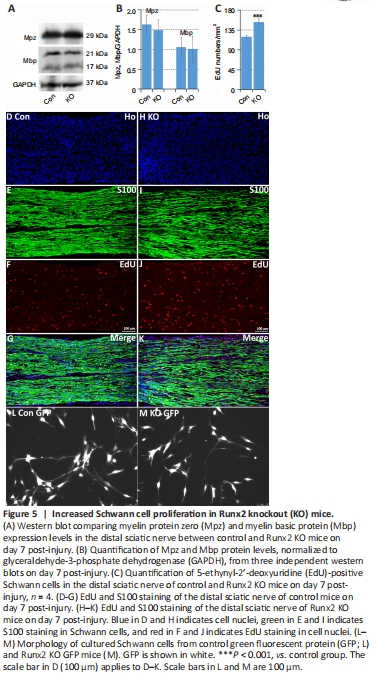
Hung et al. (2015) showed that Runx2 up-regulation in Schwann cells is controlled by c-Jun, a transcription factor that reprograms Schwann cells into a repair-competent cell type following injury (Parkinson et al., 2008; Arthur-Farraj et al., 2012). Thus, we next analyzed Schwann cell de-differentiation and proliferation in Runx2 KO mice (Parkinson et al., 2004; Arthur-Farraj et al., 2012; Gomez-Sanchez et al., 2015; Huang et al., 2015). At 7 days following sciatic nerve transection injury, we harvested the tibial nerves and examined Mpz and Mbp clearance by western blot. In contrast to the impaired myelin clearance seen in c-Jun knockout mice (Fontana et al., 2012), myelin clearance was normal in Runx2 KO mice on day 7 post-injury (Figure 5A and B). Next, we studied Schwann cell proliferation in vivo at day 7 post-injury by EdU injection. EdU/S100 double staining of longitudinal cryosections of the distal sciatic nerve (Figure 5D–K) showed that there were significantly more EdU-positive Schwann cells in the distal sciatic nerve of Runx2 KO mice than in control mice (P < 0.001; Figure 5C), which suggests that Runx2 inhibits Schwann cell proliferation following nerve crush injury.
A previous study showed that loss of c-Jun results in Schwann cell morphological changes in vitro: the cells lose their typical bipolar shape and become round and flat (Arthur-Farraj et al., 2012). When we analyzed cultured Schwann cells from adult control GFP and Runx2 KO GFP mice, we found that the Runx2 null Schwann cells still exhibited a typical bipolar shape (Figure 5L and M). The different effects of Runx2 and c-Jun on myelin clearance, cell proliferation, and morphology demonstrate that, although Runx2 is a c-Jun target (Figure 2K), these two proteins have distinct roles in Schwann cell function following injury.
Figure 6|Runx2 promotes Schwann cell migration.
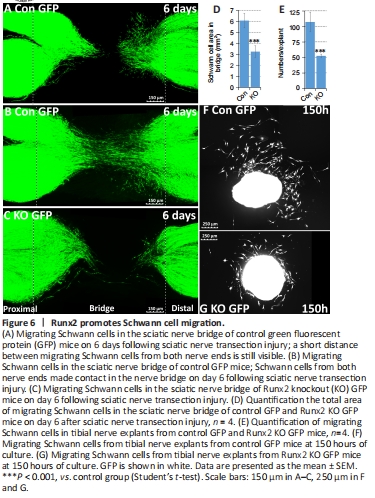
Following a nerve transection injury, Schwann cells from both the proximal and distal nerve stumps migrate into the gap, alongside nerve fibroblasts, endothelial cells, and immune cells, to form new tissue, known as the nerve bridge (Parrinello et al., 2010; Cattin et al., 2015; Chen et al., 2019; Min et al., 2020). Here, we used two approaches to study control and Runx2 KO Schwann cell migration. First, using mice with the PLP-GFP transgene, we measured Schwann cell migration following injury in control and Runx2 KO nerves in vivo. At 6 days post-transection injury, nerve and nerve bridge tissue were harvested, and samples were cleared for imaging using glycerol treatment to assess migration of GFP-positive Schwann cells into the nerve bridge (Dun and Parkinson, 2015, 2018; Chen et al., 2019; Dun et al., 2019). In control mice, large numbers of GFP-positive Schwann cells from both the proximal and distal nerve ends migrated into the bridge (Figure 6A). In some nerves from control mice, migrating Schwann cells from both nerve ends had even met in the middle of the nerve bridge at this timepoint (Figure 6B). In contrast, fewer migrating Schwann cells were observed in Runx2 KO mice at day 6 (Figure 6C). Measuring the total area of migrating Schwann cells in the nerve bridge revealed that Schwann cell migration into the nerve bridge was significantly impaired in Runx2 KO mice compared with control mice (Figure 6D). Second, we used a nerve explant culture approach that has been previously used to measure Schwann cell migration (Wang et al., 2012; Shin et al., 2017). We cultured tibial nerve explants (2 mm in length) from both control PLP-GFP mice and Runx2 KO PLP-GFP mice and examined in vitro Schwann cell migration. Live-cell images were taken after 150 hours of culturing (Figure 6F and G), and the number of GFP-positive Schwann cells migrating from each explant was counted. In line with the in vivo Schwann cell migration data, significantly fewer migrating Schwann cells were observed in Runx2 KO nerve explants comparing with control nerve explants (Figure 6E). Thus, both in vivo and in vitro approaches showed that Runx2 promotes Schwann cell migration following nerve injury.
Figure 7|Runx2 promotes axon regeneration and Schwann cell re-myelination.
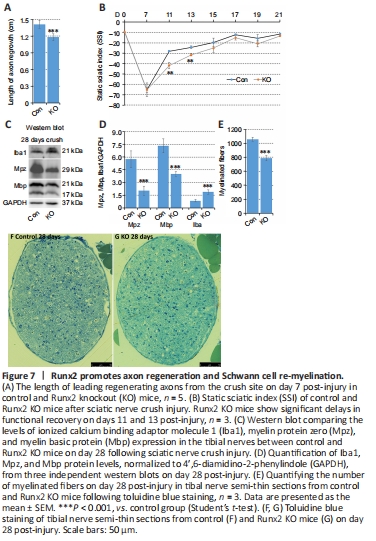
Following injury, Schwann cells play a major role in producing neurotrophins and providing a substrate for axonal regrowth. This regeneration process is best visualized by whole-mount staining using a neurofilament heavy chain antibody to identify regrowing axons (Dun and Parkinson, 2015, 2018a, b). At 7 days post-crush injury, we observed a clear and significant reduction in the axonal growth rate from the crush site in Runx2 KO animals compared with control animals (P < 0.001; Figure 7A). Next, we assessed functional recovery by SSI measurement. Before injury, as well at 7 day post-injury, there was no difference in SSI score between the control and Runx2 KO mice. However, Runx2 KO mice exhibited significantly lower SSI scores than control animals on days 11 and 13 post-injury (P < 0.05). This delay was transient, as there was no significant difference in SSI between control and Runx2 KO mice from day 15 onwards (Figure 7B). Thus, loss of Runx2 expression in Schwann cells inhibited axon regeneration and transiently delayed functional recovery following nerve crush injury.
Figure 8|Tibial nerve structure of control and Runx2 knockout (KO) mice.
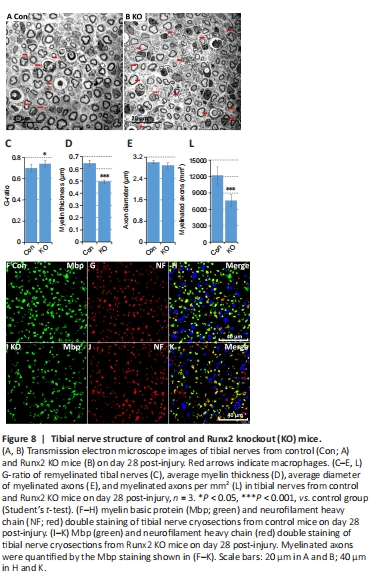
Figure 9|Normal density of regenerated axons in Runx2 knockout (KO) mice.
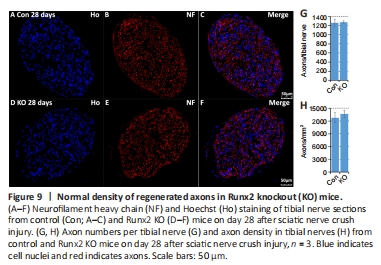
Although Runx2 is not expressed during nerve development (Figure 3F), the regulation of its expression by cAMP (Figure 2I) implies that it plays a role in re-myelination following injury. Previously, Ding et al. (2018) showed that cAMP treatment up-regulates Runx2 expression in cultured rat primary Schwann cells. We therefore assessed expression levels of the myelin proteins Mpz and Mbp in the tibial nerve by western blot on day 28 post-crush injury. The results showed that Mpz and Mbp were expressed at significantly lower levels in Runx2 KO mice compared with control mice (P < 0.001; Figure 7C and D). Next, we examined the tibial nerve structure in semi-thin sections (Figure 7F and G) and found that the number of myelinated axons in Runx2 KO nerves was significantly (P < 0.001) reduced compared with nerves from control mice (Figure 7E). We further examined tibial nerve re-myelination by TEM (Figure 8A and B) and noted significant differences in G-ratio and myelin thickness on day 28 post-crush injury between control and Runx2 KO mice (Figure 8C and D), although the diameter of the myelinated axons was similar between control and Runx2 KO mice (Figure 8E). Furthermore, there was a significant (P < 0.001) decrease in the number of myelinated axons in Runx2 KO mice compared with control mice (Figure 8L). In addition, Mbp and neurofilament heavy chain double immunostaining of tibial nerve cryosections showed reduced myelinated axon density in Runx2 KO mice (Figure 8F–K). Quantifying the number of total axons in the tibial nerve on day 28 following sciatic nerve crush injury (Figure 9A–F) showed that there was no difference in axon numbers or density between the control and Runx2 KO mice (Figure 9G and H). Thus, the reduced number of myelinated axons resulted from impaired Schwann cell re-myelination rather than a reduced number of nerve fibers.
Figure 10| Impaired macrophage clearance in Runx2 knockout (KO) mice.
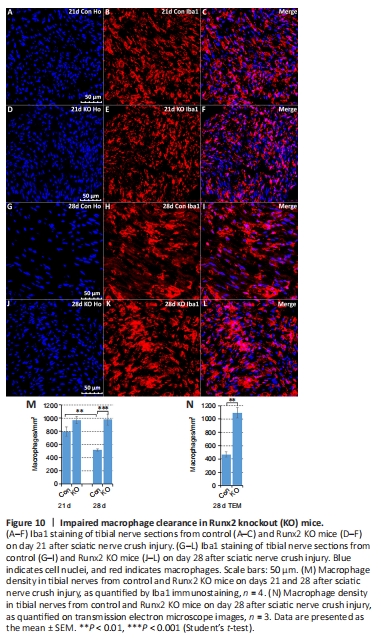
In addition to reduced re-myelination of axons in Runx2 KO mice, examination of the TEM images clearly showed that there were significantly (P < 0.05) more macrophages in the tibial nerves of Runx2 KO mice on day 28 post-crush injury compared with control mice (Figure 8A and B). Preliminary western blotting of sciatic nerve tissue on 28 days post-injury with the pan-macrophage marker Iba1 showed a significant (P < 0.001) increase in Iba1 expression in Runx2 KO mice compared with control mice (Figure 7C and D). These observations led us to carry out a more detailed exploration of macrophage recruitment and clearance in nerves by immunostaining tibial nerves for Iba1, a macrophage marker (Figure 10A–L). On day 21 post-injury there was no significant difference (P = 0.1191) in macrophage numbers between control and Runx2 KO mice (Figure 10M), indicating that macrophage recruitment was not affected in Runx2 KO mice. On day 28 post-injury, the number of macrophages was significantly (P < 0.05) reduced in the tibial nerves of control mice compared with day 21 post-injury (Figure 10M), showing rapid macrophage clearance from the tibial nerve in the fourth week of regeneration. However, macrophage clearance was impaired in Runx2 KO mice (Figure 10M and N).