脑损伤
-
Figure 3|ROS-scavenging functional hydrogels treat TBI through neuroprotection, pro-nerve regeneration, and anti-neuroinflammatory effects.
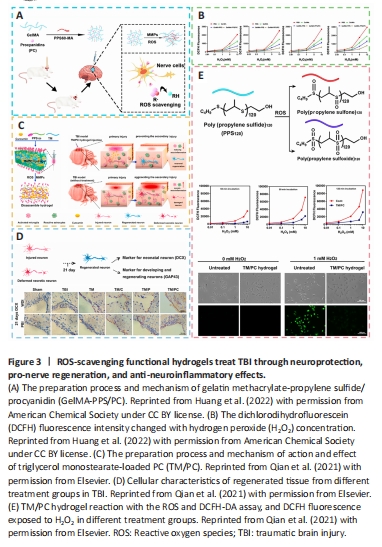
In contrast to traditional systemic drug treatment, which is currently unable to effectively deliver the medicine to the targeted region in the early stages of TBI and might instead have systemic adverse reactions owing to the existence of the BBB, gelatin methacrylate-propylene sulfide/procyanidin (GelMA-PPS/PC) has provided an immediate approach to the surface of brain tissue and concentrated on the injured region (Huang et al., 2022). As a result of the involvement of the hydrogen peroxide (H2O2)-responsive material of hydrophobic poly (propylene sulfide) 60 (PPS60), PPS60 might additionally trigger hydrogel construction to breakdown and expel the procyanidins it contains. In addition, PC could modulate the oxidative stress response in the cells, work in concert to deplete ROS, and raise the level of brain-derived neurotrophic factor (BDNF), so that could exert a neurotrophic protective function, and inhibit the expression of ionized calcium-binding adapter molecule 1 (Iba-1), glial fibrillary acidic protein, chemokine (C-X-C motif) ligand 1, tumor necrosis factor-α, interleukin-1β (IL-1β), and IL-8 to reduce inflammation (Grebenik et al., 2020). Triglycerol monostearate-loaded PC (TM/PC) was another novel ROS depletion hydrogel. According to a study, PPS120 and curcumin (Cur) might be enclosed within the hydrophobic core of TM lamellae, which could be self-assembled into a hydrogel to create a TM/PC hydrogel. Upon direct injection into the TBI wound cavity, TM decomposed, and PPS120 scavenged ROS to release Cur, thereby decreasing neural inflammation and subsequent damage spread. In addition, enhanced neuronal growth and regeneration as well as migration after TM/PC treatment could be observed by more powerful DCX expression. Moreover, a lower dichlorodihydrofluorescein fluorescence intensity after treatment showed stronger ROS-scavenging ability of the TM/PC hydrogel (Qian et al., 2021; Figure 3).
Figure 4|H2S-releasing SF hydrogel (H2S@SF hydrogel) inhibits neuronal pyroptosis and inflammation, and exerts a neuroprotective impact by inhibiting the expression of pyroptosis-related proteins, H2S synthase, and proinflammatory factors.
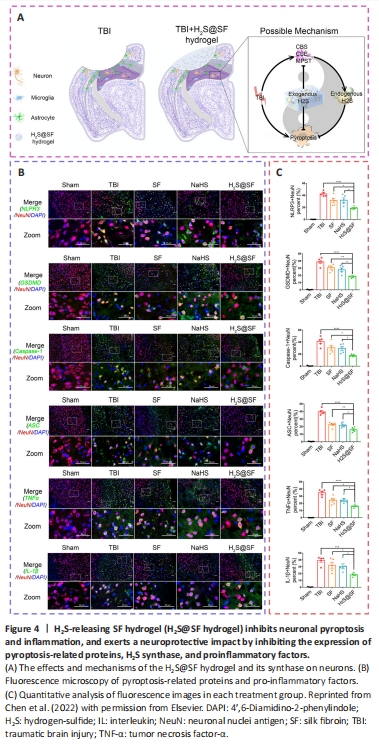
The prevention of neuroinflammation and pyroptosis was thought to be a potential therapeutic method for TBI. In recent studies, a surface-fill hydrogen-sulfide (H2S)-releasing silk fibroin (SF) hydrogel (H2S@SF hydrogel) was shown to be neuroprotective following TBI (Liu et al., 2023e). On the one hand, the H2S@SF hydrogel most significantly inhibited the activation of H2S synthase, and the expression of neuronal NOD-like receptor protein 3, gasdermin D, cysteinyl aspartate specific proteinase-1 (caspase-1), apoptosis-associated speck-like protein containing CARD, IL-1β, and tumor necrosis factor-α, and inhibited TBI-induced pyroptosis and necroptosis-related protein (receptor interacting protein-1; Wehn et al., 2021; Chen et al., 2022; López-Preza et al., 2023). H2S@SF hydrogel, on the other hand, appeared to slow the decrease in the level of glutathione following moderate TBI, demonstrating that it might protect against mitochondrial dysfunction and oxidative stress in this scenario (Kwiecien, 2021). In addition, H2S@SF could reduce neuroinflammation by inhibiting the proliferation and activation of reactive astrocytes and activated microglia, and the expression of glial fibrillary acidic protein and Iba-1 in these glial cells (Zhang et al., 2022a; Figure 4).
Figure 5|By preventing the appearance of activated astrocytes and microglia, reducing inflammatory cytokine levels, and activating M1-to-M2 macrophage reprogramming, AMEC significantly reduces oxidative stress, attenuates neural inflammation, and prevents the subsequent spread of damage following TBI.
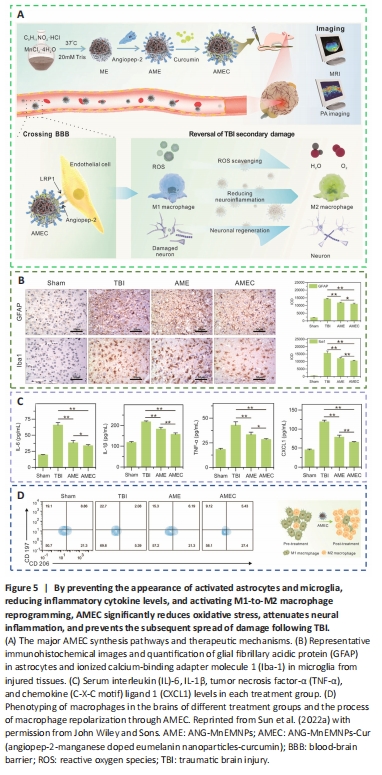
Bioactive multifunctional nanocomposites (ANG-MnEMNPs-Cur [AMEC]) can regulate antioxidation and anti-neuroinflammation for targeted TBI therapies by integrating the neuroprotective drug Cur with angiopep-2 functionalities and manganese-doped eumelanin-like nanoparticles (Fyfe, 2020; Sun et al., 2022a). Mechanistically, angiopep-2 could bind to low-density lipoprotein receptor-related protein-1 and help cross the BBB into TBI lesions and enhance drug accumulation (Bechinger et al., 2023). Melanin-like nanoparticles had powerful and extensive scavenging capabilities against an array of ROS (Zinger et al., 2021a). Cur, a specific kind of plant polyphenol, offered several pharmacological benefits, including antioxidative, anti-inflammatory, and antiapoptotic effects (Dong et al., 2022). The functional moieties of Cur and eumelanin, when combined, work cooperatively to increase the effectiveness of AMEC at the targeted site. This was accomplished by reducing oxidative stress, preventing neurological inflammation by converting M1 macrophages to M2 macrophages, and promoting neuronal regeneration (Hong et al., 2020; Wu et al., 2021; Figure 5).
Figure 7|SLanc, an angiogenic self-assembling peptide hydrogel, accelerates ischemic tissue revascularization by improving endothelial cell adhesion and angiogenesis.
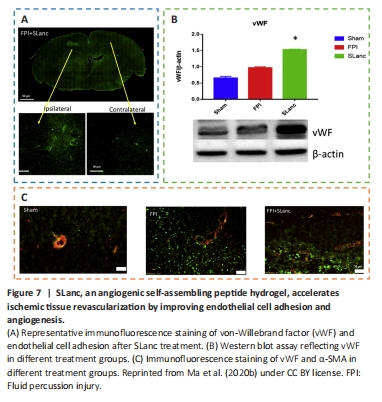
Figure 8|C/H scaffolds combined with VEGF can promote the regeneration of axons and myelin sheaths and promote angiogenesis after TBI.
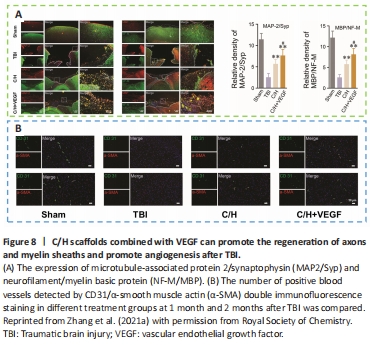
Angiogenesis initiates a vascular network in the growing body that results in the growth and maturation of the CNS (Ma et al., 2020b). Neogenesis/maturity, permeability, and the capacity to revascularize therapeutic regions of ischemia are crucial for the development of angiogenesis. SLanc, an angiogenic self-assembling peptide hydrogel, could accelerate endothelial cell attachment and angiogenesis by greatly increasing the recruitment of von-Willebrand factor, which plays an important role in CNS neuroprotection and angiogenesis (Ma et al., 2020b; Figure 7). The most important angiogenesis-inducing growth factor, vascular endothelial growth factor (VEGF), is associated with increased BBB opening, brain edema, and neurological impairments. In a recent study, a VEGF-containing hydrogel was injected directly into the region of damage to stimulate the creation of a vascular and neuronal structure that led to behavioral improvement (Zhang et al., 2021a). In the injured area, HA gel and high cluster VEGF might encourage the development of a strong, mature, and well-established vascular bed and accelerate axonal extention alongside these blood vessels. Additionally, this hydrogel could encourage the inward migration of embryonic neurons from the subventricular zone, decrease the activation of microglia and the extent of the reactive astrocyte boundary, and change the surrounding peri-infarct tissue (Ma et al., 2020a). Meanwhile, numerous studies have proven that heparin exerted anti-inflammatory effects by blocking leukocyte adhesion to the vessel wall and stabilizing chemokine and growth factor gradients (Schirmer et al., 2020; Zhang et al., 2021a; Liu et al., 2022b). VEGF delivery was related to increased inflammation, which could weaken the efficacy and strengthen the adverse effects. Surprisingly, the proinflammatory action of VEGF could be counteracted by bare heparin particles, which also provided a pro-repair milieu that promoted the proliferation of fresh neural tissue (Jiang et al., 2020; Figure 8). Hence, the combination of the rapid development of a neurovascular network in the damaged location and the control of responses to inflammation, scars, and NSC in the neighboring brain position might pave the way for a new approach in the field of neural healing following TBI (Nih et al., 2018).
Figure 9|The encapsulation of BDNF-MSCs in biomaterials can protect transplanted cells from immune rejection, offer a favorable milieu, and improve the survival rate of transplanted cells at the injured site.
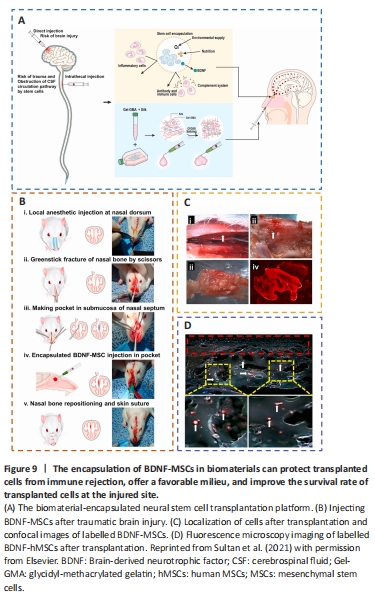
Mesenchymal stem cells (MSCs) are capable of differentiating into neurons, replacing injured cells, and secreting paracrine hormones, making them a viable therapy for brain injury (Xu et al., 2020a; Sharma et al., 2021; Viet et al., 2022; Table 2). However, there has been little success and many problems with MSCs, namely limited cell availability, short cellular lifespan, few differentiated neurons, and poor functional integration (Bonsack et al., 2020; Ruppert et al., 2020; Jha et al., 2021; Pischiutta et al., 2021). Thus, encapsulation of transplanted cells with biomaterials might provide a suitable milieu for the growth and proliferation of these cells, protect them from immune rejection, and improve cell engraftment and survival during transplantation of injured brain lesions (Sultan et al., 2021; Figure 9).
Figure 10|The implantation of regenerative complexes could strengthen the reconstruction of neural networks and the recovery of motor function after TBI.
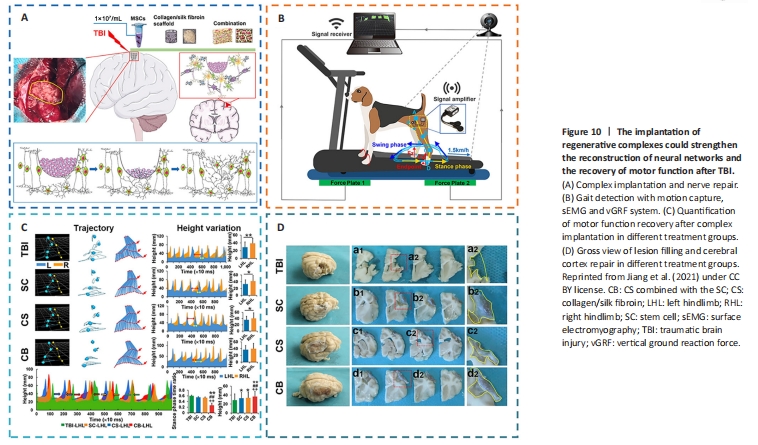
To improve the rate of cell engraftment and survival, some scholars developed a new thermosensitive hydrogel based on chitosan, hydroxyethyl cellulose, HA, and β-glycerophosphate (CS-HEC-HA/GP), which possessed better biocompatibility with human umbilical cord mesenchymal stem cells (hUC-MSCs) and a faster gelation process than traditional hydrogel (Yao et al., 2019a; Yea et al., 2020). In comparison to BMSCs, hUC-MSCs offered the benefits of abundant sources, simple accessibility, few negative outcomes for the donor, low requirement for consistency in human leukocyte antigen matching, and less danger of viral infection following transplantation (Chen et al., 2020a; Barretto et al., 2021). CS-HEC-HA/GP hydrogel-laden hUC-MSCs had the ability to enhance the reservation, survival, and transfer of encapsulated hUC-MSCs (Sultan et al., 2021). Moreover, this synthesized hydrogel could possibly secrete neurotrophic factors and suppress apoptosis to promote the subsistence and multiplication of endogenous neurons (Torres-Ortega et al., 2022; Zhang et al., 2023), and expedite the rebuilding of brain tissue and recovery of neural function in TBI patients (Liu et al., 2020a, 2023b; Bamshad et al., 2023). Jiang et al. (2021) reported that compared with the simple stem cell (SC) or collagen/SF (CS) scaffold, the implantation of CS combined with the SC (CB) scaffold showed better cortical integrity and motor function and more regeneration of nerve fibers (Figure 10).