周围神经损伤
-
Figure 4 | In situ hybridization spatial mRNA validation of Ezh2 expression in sciatic nerve of rats.
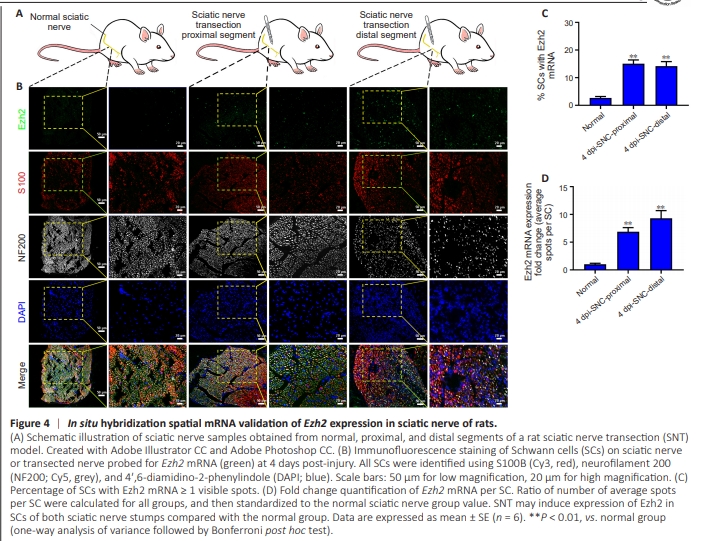
To confirm sciatic nerve tissue localization of Ezh2 expression in normal and injured sciatic nerve segments (Figure 4A), insitu hybridization was used to examine anticipated changes in gene expression of EZH2 (green) in S100-positive SCs. Along with Ezh2 transcripts exhibiting co-localization with S100 immunostaining in normal sciatic nerve, upregulated Ezh2 colocalized with S100-positive SCs in proximal and distal nerve segments at 4 days post-SNT (Figure 4B). The percentage of SCs with Ezh2 expression was significantly higher at 4 days after SNT in proximal and distal trunks than in normal sciatic nerves (Figure 4C). Moreover, the copy number of Ezh2 mRNA expression per SC was higher in the sciatic nerve trunk at 4 days after SNT compared with the control group (Figure 4D). All data indicate that SNT may induce Ezh2 expression in SCs of rats with injured sciatic nerves, in accordance with our previous microarray data (Figure 3A, B, and D).
Figure 5 | Ablation of Ezh2 in Schwann cells of mice leads to abnormal growth and myelination deficits in the sciatic nerve.
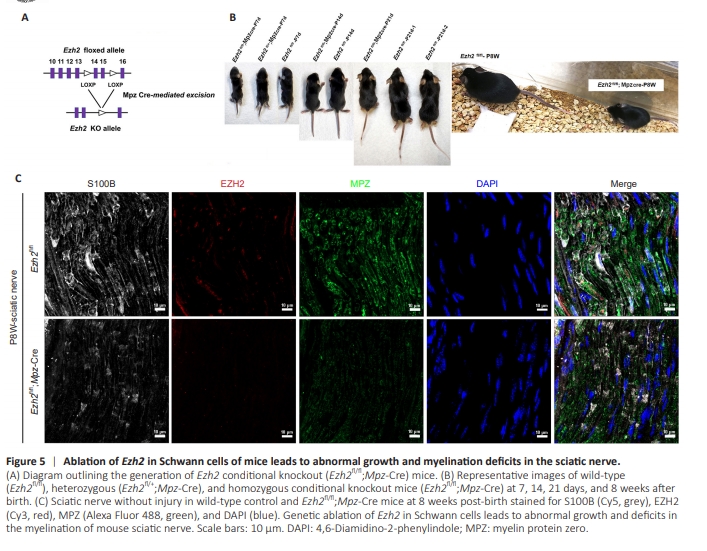
Since mice are the most common genetic model animals, we chose to generate Ezh2 cKO mice instead of rats. Typically, genetic manipulations and tools are easier using mice than rats. To investigate the function of EZH2, we specifically deleted Ezh2 in SCs by crossing Cre recombinase-expressing mice with mice carrying a Ezh2 floxed allele under control of a Mpz promoter (Mpz:Ezh2fl/fl) (Figure 5A). This promoter triggers Cre expression from embryonic day (E)13.5, prior to sorting of the axes or differentiation of fetal stem cells (Rosenberg et al., 2018). We compared Ezh2 cKO mice (Ezh2fl/fl; Mpz-Cre) with their littermate wild-type controls (Ezh2fl/fl). Mutant mice grew abnormally and initially showed a visibly smaller body size and weight. Mice at 8 weeks post-birth (P8W) began to experience severe weakness and motor deficits associated with muscle loss. These symptoms indicate that expression of Ezh2 in SCs is potentially required for proper neural function and SC development. Representative images of wild-type (Ezh2fl/fl), heterozygous (Ezh2fl/+;Mpz-Cre), and homozygous cKO mice (Ezh2fl/fl;Mpz-Cre) are shown at 7, 14, 21 days, and 8 weeks after birth (Figure 5B). Representative validation confirmed the success of the Ezh2 construct in generating cKO mice (Figure 5C). These data suggest that genetic ablation of Ezh2 in SCs of mice leads to abnormal growth and deficits in myelination of the sciatic nerve.
Figure 6 | Knockout of Ezh2 in Schwann cells leads to hypomyelination of mouse sciatic nerve and suppresses remyelination post-SNC.
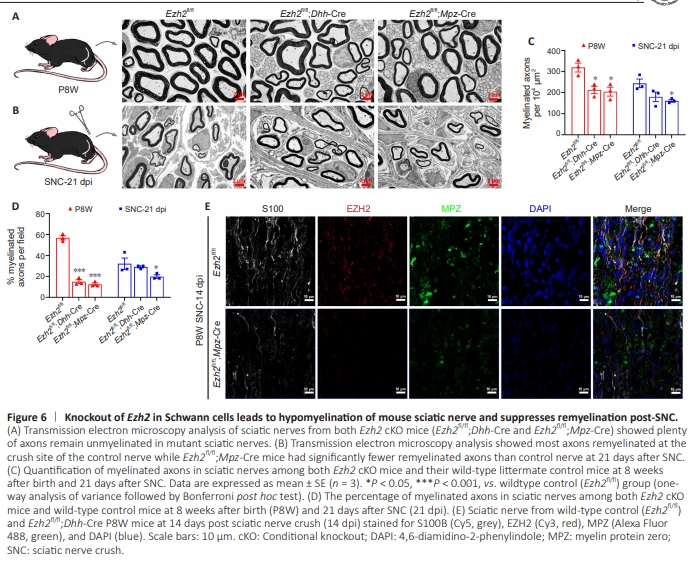
To specifically delete the Ezh2 gene in SCs, we generated transgenic mice expressing the Cre recombinase from regulatory sequences of Dhh (Chen et al., 2011). The Dhh gene is highly expressed in precursors of SCs during nervous system development and in Sertoli cells of the testis (Bitgood and McMahon, 1995). Dhh-Cre activity was observed in the SC lineage from E12 onwards, as shown by blue staining of the peripheral nerve (Wu et al., 2008; Guo et al., 2013; Miyamoto et al., 2018). Dhh-Cre activity is also observed in the testis, which is another well-documented site of Dhh gene expression. In addition to the SC lineage and the testis, Cre expression is observed in the skin of the snout (but not the brain), specifically in the hypothalamus region (Jaegle et al., 2003). Hence, Dhh was chosen to construct cKO mice in the present work.We crossed Ezh2-floxed mice (Ezh2fl/fl) with the SC lineage expressing Dhh-Cre line to eliminate the Ezh2 gene (Ezh2fl/fl; Dhh-Cre). Similar to Ezh2fl/fl;Mpz-Cre mice, all Ezh2fl/fl;Dhh-Cre mice survived for more than 8 weeks. Electron microscopy (EM) analysis of the sciatic nerves in both Ezh2 cKO mouse lines (Ezh2fl/fl;Dhh-Cre and Ezh2fl/fl;Mpz-Cre) showed that many axons remained unmyelinated in mutant sciatic nerves. Indeed, it has been suggested that Ezh2 is necessary for the proper initiation of SC myelination (Figure 6A, C, and D). As previously stated, Ezh2 is minimally expressed in adult sciatic nerves but significantly upregulated during SC regeneration following PNI. To investigate the necessity of Ezh2 in SC remyelination, we performed a SNC model on both types of adult Ezh2 cKO mice and their wild-type littermates. For histological analysis, we collected sciatic nerves at 21 days after axotomy (21 dpi). EM analysis showed that at 21 days after SNC, most axons were remyelinated at the crush site of the control nerve. Meanwhile, Ezh2fl/fl;Mpz-Cre mice had significantly fewer remyelinated axons than control nerves (Figure 6B–D). These data indicate that Ezh2 may be essential for improving SC remyelination during peripheral nerve regeneration. Representative validation on knocking-out Ezh2 in SCs (Ezh2fl/fl;Dhh-Cre) led to hypomyelination of mouse sciatic nerve and suppressed remyelination at 14 days postSNC (Figure 6E).