周围神经损伤
-
Figure 1 | pDNM-gel improves nerve regeneration and functional restoration in vivo.
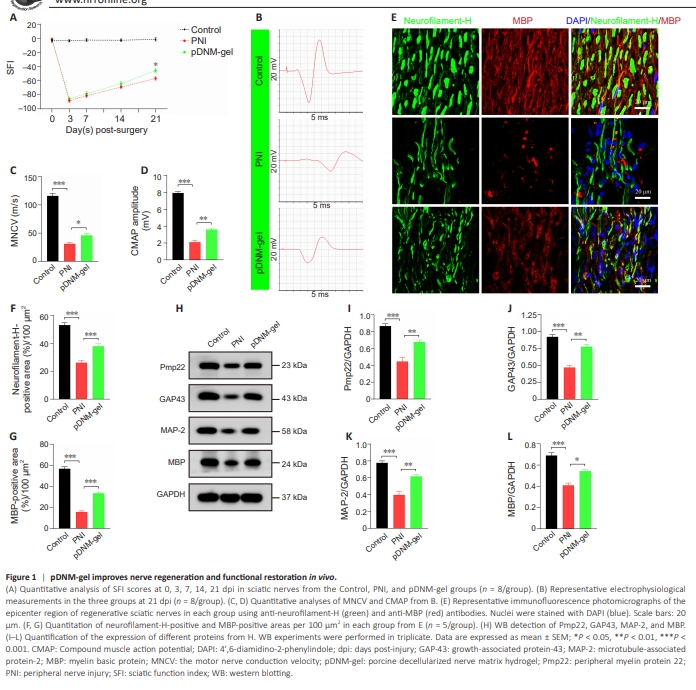
First, we investigated the therapeutic effect of pDNM-gel on peripheral nerve recovery in terms of locomotor function, nerve conduction and structure, and related molecular changes. A walking track analysis was performed to obtain SFI values at 0, 3, 7, 14, and 21 days post-surgery. As shown in Figure 1A, all rats displayed normal pre-operative sciatic nerve function, but exhibited several hindlimb paralyses after PNI. Over the next few weeks, their locomotor function gradually improved. At 21 dpi in particular, model rats in the pDNMgel group had significantly higher SFI values than those in the PNI group (P < 0.05; Figure 1A). Electrophysiological analysis revealed that, compared with the PNI group, the pDNM-gel group exhibited faster nerve conduction velocity (P < 0.05 for motor nerve conduction velocity; Figure 1B and C) and better recovery of the gastrocnemius muscle (P < 0.01 for CAMP; Figure 1B and D). Next, we co-stained the sciatic nerve tissue sections with the axon marker neurofilament-H and the myelin marker MBP. Immunological staining of the epicenter regions of the regenerated nerves showed that immunoreactivity to neurofilament-H and MBP in the pDNM-gel group was significantly stronger compared with that in the PNI group, but was weaker than that in the Control group (Figure 1E). Statistical analysis further revealed that the percentage of the area positive for neurofilament-H and MBP staining was significantly larger in the pDNM-gel group than in the PNI group (P < 0.001; Figure 1F and G). Similarly, there were more areas of positivity for neurofilament-H and MBP in both the proximal and distal sites of the injured nerves in the pDNMgel group than in the PNI group, suggesting more effective repair of injured nerves under pDNM-gel treatment (P < 0.001; Additional Figures 4 and 5). The levels of growth regulation and remyelination-related proteins, namely Pmp22, GAP43, MAP-2, and MBP, were dramatically higher in regenerated nerves from the pDNM-gel group than in those from the PNI group (P < 0.01 for Pmp22, GAP43, and MAP-2; P < 0.05 for MBP; Figure 1H–L).
Figure 2 | pDNM-gel attenuates M1 macrophage activation and forms an anti-inflammatory microenvironment to promote peripheral nerve regeneration.
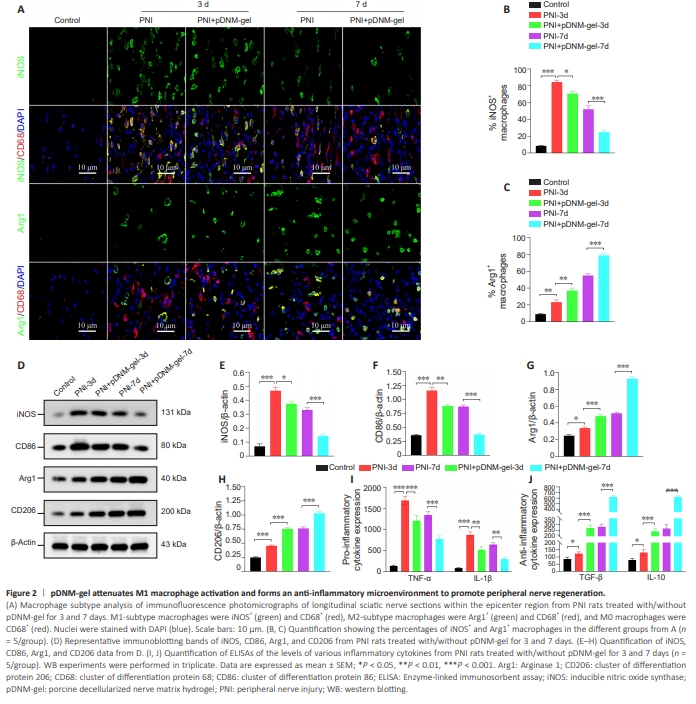
Next, we evaluated the regulatory effects of pDNM-gel on macrophage polarization within the epicenter region of injured sciatic nerves at 3 and 7 dpi. Immunofluorescence analysis at each time point following PNI showed that, as the number of iNOS+ CD68+ M1-type macrophages gradually decreased, the number of Arg1+ CD68+ M2-type macrophages remarkably increased (Figure 2A). Under the administration of pDNM-gel, this phenotypictransformation trend was more pronounced (Figure 2A). Consistently, the percentage of iNOS+ macrophages was lower in pDNM-gel group rats than in PNI group rats (P < 0.05 at 3 dpi; P < 0.001 at 7 dpi; Figure 2B), while the percentage of Arg1+ macrophages in these two groups showed the opposite trend (P < 0.01 at 3 dpi; P < 0.001 at 7 dpi; Figure 2C). Furthermore, the trends in macrophage polarization at the proximal and distal sites of the injured nerves were consistent with those at the epicenter region. Specifically, samples from PNI rats with or without pDNM-gel treatment had a higher percentage of M2-subtype macrophages at 7 dpi than at 3 dpi, and there were more M2 macrophages in pDNM-gel rats than in PNI rats at the same time points (Additional Figures 6 and 7). In WB analysis, the patterns of changes in the expression of M1 phenotypic markers (iNOS and CD86) and M2 phenotypic markers (Arg1 and CD206) between the PNI and pDNM-gel groups were similar to those in immunofluorescence analysis (Figure 2D). Specifically, at 3 dpi, PNI rats treated with pDNM-gel showed decreased protein levels of M1 macrophage markers compared with the PNI group (P < 0.05 for iNOS; P < 0.01 for CD86; Figure 2E and F) and increased protein levels of M2 macrophage markers compared with the PNI group (P < 0.001 for Arg1; P < 0.001 for CD206; Figure 2G and H). At 7 dpi, these differences were even more apparent (pDNM-gel group versus PNI group: P < 0.001 for iNOS, CD86, Arg1, and CD206; Figure 2E–H). We also detected the levels of inflammatory cytokines at 3 and 7 dpi using ELISAs. As depicted in Figure 2I and J, the indicated inflammatory cytokines were upregulated at the early stage in all PNI rats, but were altered to varying degrees by pDNM-gel treatment. Specifically, the concentrations of proinflammatory cytokines TNF-α and IL-1β were significantly lower in PNI + pDNM-gel rats than in PNI rats (TNF-α: P < 0.001 at 3 dpi and P < 0.001 at 7 dpi; IL-1β: P < 0.01 at 3 dpi and P < 0.01 at 7 dpi; Figure 2I). In contrast, the concentrations of anti-inflammatory cytokines TGF-β and IL-10 were significantly higher in PNI + pDNM-gel rats than in PNI rats (TGF-β: P < 0.001 at 3 dpi and P < 0.001 at 7 dpi; IL-1β: P < 0.001 at 3 dpi and P < 0.001 at 7 dpi; Figure 2J).
Figure 4 | pDNM-gel inhibits M1 macrophage polarization and pro-inflammatory cytokine production in vitro.
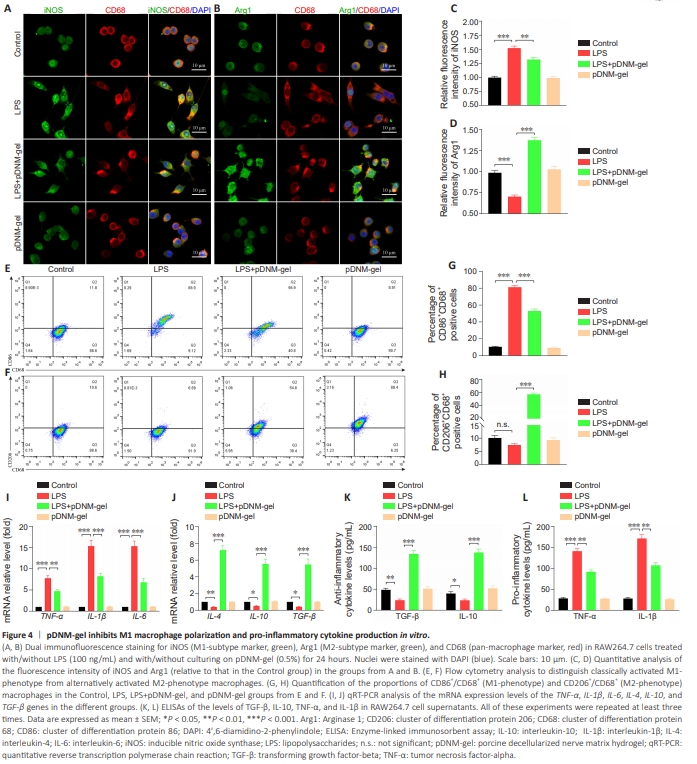
To demonstrate that pDNM-gel could also accelerate M2 polarization of macrophages in vitro, we employed the immortalized murine RAW264.7 macrophage cell line, stimulated by LPS, with or without administration of pDNM-gel. Cell polarization and cytokine accumulation were then detected via immunofluorescence staining, flow cytometry, qRT-PCR, and ELISA. Immunofluorescence staining images showed that LPS induced abundant iNOS expression and blocked Arg1 production, but the reverse effect wasobserved when LPS-stimulating RAW264.7 cells were grown on the pDNMgel surface (LPS + pDNM-gel group versus LPS group: P < 0.01 for iNOS immunoreactivity and P < 0.001 for Arg1 immunoreactivity; Figure 4A–D). Similarly, flow cytometry analysis demonstrated that culturing on pDNMgel increased the percentage of M2 phenotype cells and decreased the percentage of M1 phenotype cells under LPS-stimulation (LPS + pDNM-gel group versus LPS group: P < 0.001 for CD86+ /CD68+ cells and P < 0.001 for CD206+ /CD68+ cells; Figure 4E–H). The levels of pro- and anti-inflammatory genes and cytokines were evaluated to detect the classical features of macrophage polarization and their corresponding impacts. qRT-PCR analysis showed that LPS stimulation of RAW264.7 cells largely activated M1-like phenotype genes (Figure 4I). Specifically, expression levels of the TNF-α and IL-1β genes increased by nearly 8-fold, while those of the IL-6 gene increased by approximately 15- fold (LPS group versus Control group: P < 0.001). By contrast, under pDNMgel protection, LPS-stimulated RAW264.7 cells showed upregulation of M2- related phenotype genes, including IL-4, IL-10, and TGF-β (LPS + pDNM-gel versus LPS group: P < 0.001 for IL-4, IL-10, and TGF-β; Figure 4J). In agreement with the qRT-PCR analysis, ELISA data also showed that, compared with LPS-stimulated RAW264.7 cells, those treated with LPS and cultured on pDNM-gel exhibited significantly enhanced production of antiinflammatory cytokines (P < 0.001 for TGF-β and IL-10; Figure 4K), and notably restricted secretion of pro-inflammatory cytokines (P < 0.01 for TNF-α and IL-1β; Figure 4L).
Figure 5 | Changes in expression of TLR4/MyD88/NF-κB signaling pathway components in RAW264.7 cells cultured on pDNM-gel and stimulated by LPS.
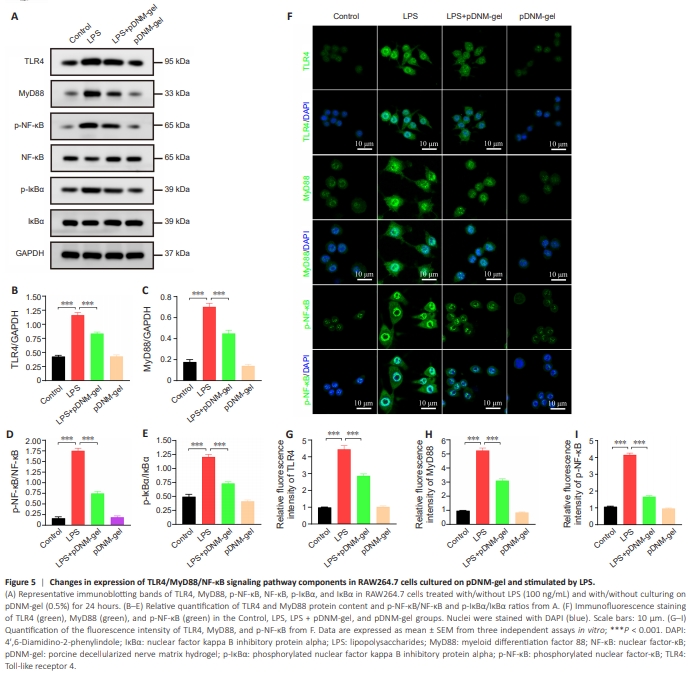
As a canonical inflammatory transcriptional pathway, the TLR4/MyD88/ NF-κB axis is essential for inducing M1 macrophage polarization and proinflammatory cytokine secretion (Abdollahi et al., 2023; Geng et al., 2023). Thus, we speculated that pDNM-gel-mediated activation, polarization, and functioning of macrophages might be involved in arresting this pathway. To confirm this hypothesis, we employed WB and immunofluorescence staining to detect the influence of pDNM-gel on this pathway in RAW264.7 cells. WB results showed that the protein levels of TLR4 and MyD88, and the phosphorylation of NF-κB and IκBα, were significantly induced by LPS stimulation, while pre-coating culture wells with pDNM-gel reduced these effects (Figure 5A). Quantitative analysis also revealed that the ratios of TLR4 to GAPDH, MyD88 to GAPDH, p-NF-κB to NF-κB, and p-IκBα to IκBα weremarkedly lower in the LPS + pDNM-gel group than in the LPS group (P < 0.001 for TLR4/GAPDH, MyD88/GAPDH, p-NF-κB/NF-κB, and p-IκBα/IκBα; Figure 5B–E). Similarly, immunofluorescence analysis of LPS-stimulated RAW264.7 cells revealed that culturing on pDNM-gel resulted in markedly lower percentages of positivity for TLR4, MyD88, and p-NF-κB (Figure 5F–I; LPS + pDNM-gel group versus LPS group: P < 0.001 for TLR4, MyD88, and p-NF-κB immunoreactivities). These results confirmed that pDNM-gel could suppress the activity of the TLR4/MyD88/NF-κB axis in LPS-stimulated RAW264.7 cells.
Figure 6 | TLR4/MyD88/NF-κB pathway plays an indispensable role in regulating pDNM-gel-mediated macrophage polarization and anti-inflammatory reactions in vitro.
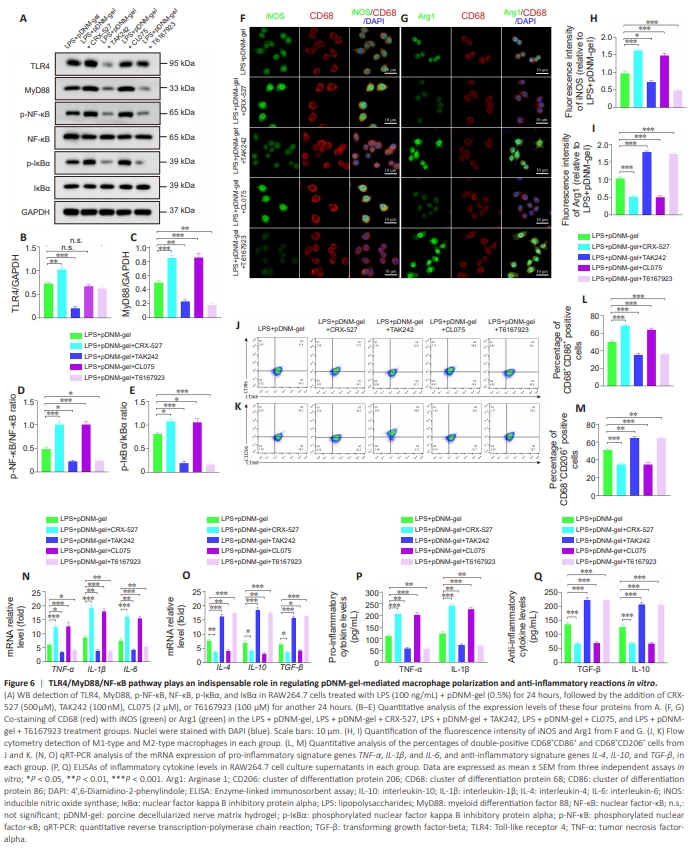
Under these treatment conditions, we found that the TLR4 agonist CRX-527 and the TLR4 inhibitor TAK242 increased and decreased the levels of TLR4 and its downstream molecules, including MyD88, p-NF-κB, and p-IκBα, respectively (Figure 6A– E). Similarly, the MyD88 agonist CL075 and the MyD88 inhibitor T6167923 promoted and inhibited the levels of MyD88, p-NF-κB, and p-IκBα, respectively (Figure 6A–E). Subsequently, we examined macrophage polarization in each group via co-immunostaining and flow cytometry. The addition of the agonists CRX-527 and CL075 increased the fluorescence intensity of iNOS and reduced that of Arg1 (Figure 6F–I), while the addition of inhibitors TAK242 and T6167923 had a tendency for the opposite effect (Figure 6F–I). The flow cytometry assays of the percentages of M1-like macrophages (CD86+ CD68+ ) and M2-like macrophages (CD206+ CD68+ ) were consistent with the results of co-immunostaining (Figure 6J–M). Finally, qRT-PCR and ELISA analyses showed that stimulation with CRX-527 and CL075 significantly upregulated the expression of pro-inflammatory-related genes (Figure 6N) and the secretion of corresponding cytokines (Figure 6P), while downregulating the expression of anti-inflammatory-related genes (Figure 6O) and the secretion of anti-inflammatory cytokines (Figure 6Q). These effects were reversed in RAW264.7 cells treated with TAK242 and T6167923 (Figure 6N–Q).
Figure 7 | Promotional effect of pDNM-gel on nerve regeneration is abolished using TLR4/MyD88/NF-κB agonists in vivo.
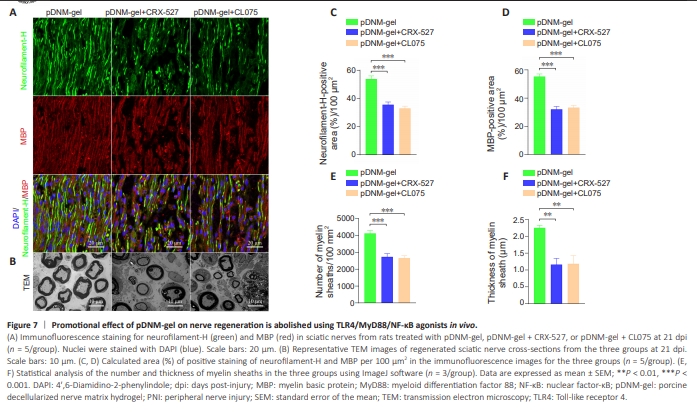
Next, we sought to examine the effects of these agonists and inhibitors of TLR4/MyD88/NF-κB signaling in vivo, using immunofluorescence and TEM. Immunofluorescence analysis showed that the positive effects of pDNM-gel on axonal and myelin regeneration were markedly suppressed by treatment of rats with CRX-527 or CL075 (Figure 7A). Statistical analysis revealed larger areas of positive staining for neurofilament-H and MBP in sciatic nerve sections of rats treated with pDNM-gel compared with those treated with pDNM-gel plus CRX527 or CL075 at 21 dpi (Figure 7C and D; neurofilament-H: P < 0.001; MBP: P < 0.001). Consistently, TEM of the ultrastructure of sciatic nerve tissues showed that both agonists significantly increased the number and thickness of newborn myelin sheaths in nerve-contused rats receiving pDNM-gel treatment (Figure 7B, E, and F; number of myelin sheaths: P < 0.001; thickness of myelin sheaths: P < 0.01).