中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (7): 2038-2052.doi: 10.4103/NRR.NRR-D-23-01265
NOX4促进神经元铁死亡和神经炎症加重帕金森病的病理改变
NOX4 exacerbates Parkinson’s disease pathology by promoting neuronal ferroptosis and neuroinflammation
Zhihao Lin, Changzhou Ying, Xiaoli Si, Naijia Xue, Yi Liu, Ran Zheng, Ying Chen, Jiali Pu* , Baorong Zhang*
- Department of Neurology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, China
摘要:
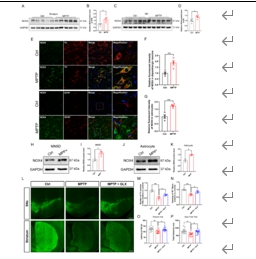
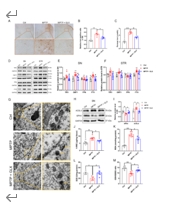
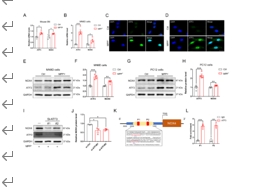
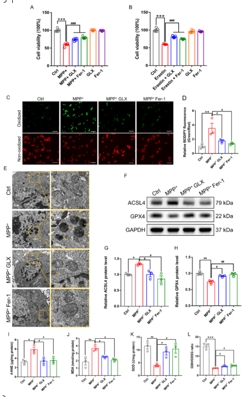
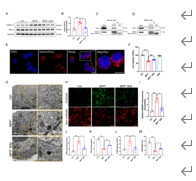
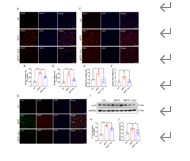
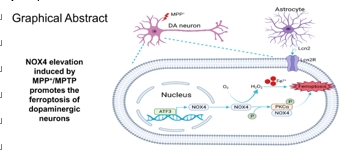
帕金森病主要是由黑质中多巴胺能神经元的丧失引起的。铁突变是一种新型的调节性细胞死亡形式,其特点是铁积累和脂质过氧化,在多巴胺能神经元的死亡中起着至关重要的作用。然而,多巴胺能神经元铁中毒的分子机制尚未完全阐明。NADPH 氧化酶 4 与氧化应激有关,但它是否调控多巴胺能神经元的铁氧化作用仍不得而知。本研究的目的是确定 NADPH 氧化酶 4 是否参与了多巴胺能神经元的铁突变,如果是,参与的机制是什么。我们发现,在 1-甲基-4-苯基-1,2,3,6 四氢吡啶诱导的帕金森病模型中,转录调节因子活化转录因子 3 增加了多巴胺能神经元和星形胶质细胞中 NADPH 氧化酶 4 的表达。抑制 NADPH 氧化酶 4 可改善帕金森病模型动物的行为障碍,减少多巴胺能神经元的死亡。此外,抑制 NADPH 氧化酶 4 还能减少帕金森病模型动物黑质中的脂质过氧化和铁积累。从机理上讲,我们发现 NADPH 氧化酶 4 与活化的蛋白激酶 C α 相互作用,阻止了多巴胺能神经元的铁沉积。此外,通过降低星形胶质细胞脂褐素-2的表达,NADPH氧化酶4抑制剂可减少1-甲基-4-苯基-1,2,3,6四氢吡啶诱导的神经炎症。这些研究结果表明,NADPH 氧化酶 4 可促进多巴胺能神经元的铁变态反应和神经炎症,从而导致多巴胺能神经元死亡,这表明 NADPH 氧化酶 4 可能是帕金森病的治疗靶点。
https://orcid.org/0000-0002-8099-7407 (Baorong Zhang); https://orcid.org/0000-0002-6719-4060 (Jiali Pu)