中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (5): 1416-1430.doi: 10.4103/NRR.NRR-D-23-01495
诱导神经干细胞调节闭合性颅脑损伤小鼠小胶质细胞活化
Induced neural stem cells regulate microglial activation through Akt-mediated upregulation of CXCR4 and Crry in a mouse model of closed head injury.
- 1 Department of Neurosurgery, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan Province, China;2 Department of Neurosurgery, Chinese PLA General Hospital, Beijing, China; 3 Department of Neurology, Fu Xing Hospital, Capital Medical University, Beijing, China
摘要:
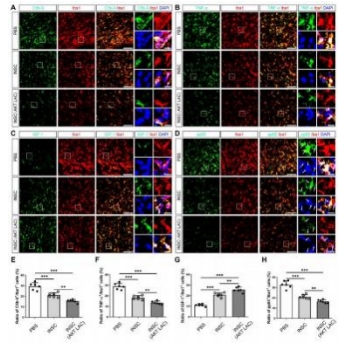
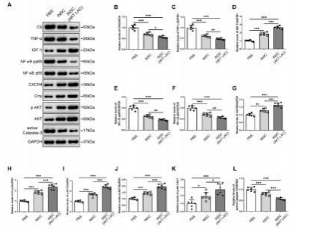
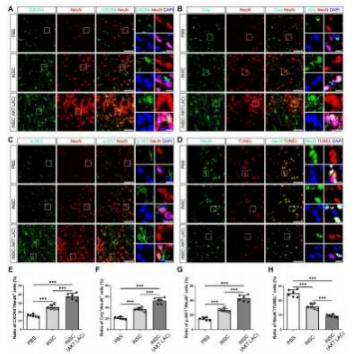
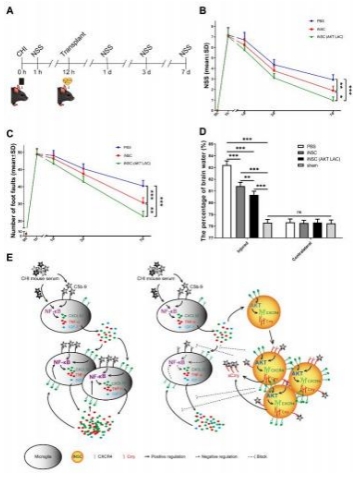
闭合性颅脑损伤后迅速活化的小胶质细胞在神经炎症介导的神经损伤和修复中发挥着重要而复杂的作用。作者既往研究显示,诱导神经干细胞可通过上调CXCR4影响小胶质细胞活化,促进神经修复。为阐明CXCR4上调机制,实验通过补体沉积、干细胞转染、细胞共培养检测结果显示,闭合性颅脑损伤模型小鼠血清可诱导小胶质细胞NF-κB活化,促其表达CXCL12和肿瘤坏死因子α,而抑制其表达胰岛素样生长因子1;然而,CR2-Crry减轻了闭合性颅脑损伤小鼠血清预处理的小胶质细胞NF-κB活化,以及CXCL12和肿瘤坏死因子α表达。诱导神经干细胞可以接受刺激(例如:小胶质细胞分泌的CXCL12),并通过CXCL12/CXCR4、Crry和Akt通路的相互作用上调CXCR4和Crry,从而调控小胶质细胞活化。与体外实验结果一致,激活诱导神经干细胞中Akt通路一方面增强了诱导神经干细胞对闭合性颅脑损伤小鼠脑损伤区小胶质细胞的调控作用,促使活化的小胶质细胞分泌胰岛素样生长因子1以促进神经修复,另一方面通过上调闭合性颅脑损伤小鼠脑损伤区CXCR4和Crry增强诱导神经干细胞的神经保护作用。激活诱导神经干细胞中Akt通路增强了诱导神经干细胞减轻闭合性颅脑损伤后神经损伤、脑水肿和神经功能障碍的疗效。结果表明Akt通路可以调节诱导神经干细胞中CXCR4和Crry表达,而CXCL12/CXCR4和Crry可以反馈调节Akt通路。值得注意的是,CXCL12/CXCR4、Crry和Akt通路之间的相互作用可能构成一个调控体系,以使CXCR4和Crry不断升高,从而阐明诱导神经干细胞调控小胶质细胞中CXCR4上调的机制。
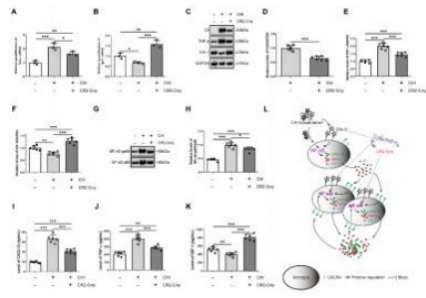 #br#
#br#
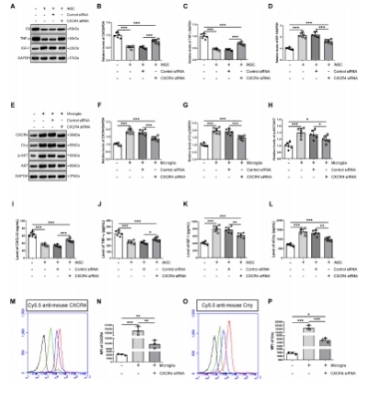 #br#
#br#
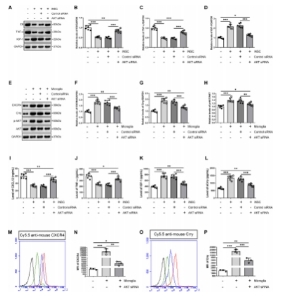 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#
 #br#
#br#