NRR:兰州大学/首都医科大学的王永刚教授团队认为海马齿状回过表达Rbm8a基因可以改善阿尔茨海默病的认知功能
阿尔茨海默病(Alzheimer’s Disease, AD)是一种神经退行性疾病,属于最常见的一种老年性痴呆,主要表现为记忆功能下降和认知功能损害,以淀粉样蛋白异常沉积形成老年斑块和tau蛋白过度磷酸化形成神经原纤维缠结为主要病理改变。其病理改变严重损害了神经元健康,造成大量神经元的结构破坏和功能丧失。有研究发现,大脑的侧脑室室下区(subventricular zone, SVZ)和海马齿状回颗粒下区(subgranular zone, SGZ)存在新生神经元,神经新生在AD疾病中明显下降[1]。所以,通过进一步加强神经元新生,有助于替代功能受损的神经细胞,为神经退行性疾病(包括AD)提供新的治疗靶点。既往研究发现, Rbm8a基因过表达可以促进胚胎神经前体细胞和视网膜祖细胞增殖,而其表达缺陷会增加神经发育性疾病的风险,如血小板减少伴桡骨缺失综合征、小头畸形[2-4]。但是,Rbm8a基因是否可以调控神经元新生,进而改善AD疾病的认知功能,尚不明确。
近期,兰州大学/首都医科大学王永刚团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Rbm8a regulates neurogenesis and reduces Alzheimer’s disease-associated pathology in the dentate gyrus of 5×FAD mice”的研究论文。通过研究,作者发现,Rbm8a基因过表达可通过促进神经前体细胞增殖,进而增加海马齿状回神经元新生,抑制了淀粉样蛋白沉积和老年斑块形成,同时缓解了神经胶质增生反应,以及营养不良神经突起的形成,从而改善了AD的记忆功能。该文的启示:Rbm8a基因调控神经新生,为AD的治疗提供了新的靶点。朱辰路博士和任潇博士为该文的共同第一作者;王永刚教授和刘亚巍教授为该文的共同通讯作者; 刘晨医生为该文的第二作者。
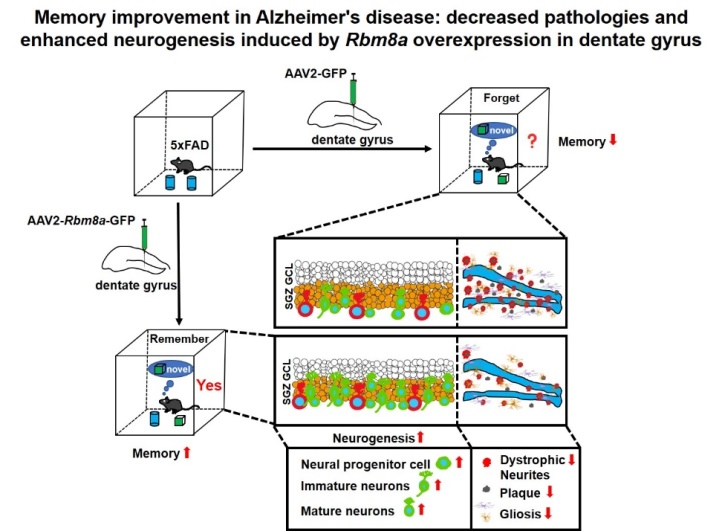
AD作为一种神经退行性病变,伴发脑皮层和齿状回的大量神经元死亡和神经新生能力下降。而有研究发现,Rbm8a基因表达与神经干细胞增殖相关[3,4]。那么,Rbm8a基因表达量是否随着年龄增长改变。作者通过评估Rbm8a基因表达量发现,其随着年龄的增长出现明显的下降趋势(图1),这与神经新生的年龄依赖性下降一致。所以,作者假说:恢复Rbm8a的表达可以促进神经新生。为了验证这个假说,作者通过病毒复制恢复Rbm8a基因在海马齿状回的表达,结果发现新生神经元明显增多(图2),AD中的病理改变减轻,包括老年斑块(图3)、营养不良神经突起(图4)。RNA测序发现,大量差异表达的基因富集在生物过程的通路中,包括神经新生,淀粉样蛋白形成和细胞增殖(图5)。并且,作者的测序结果表明,Adora2a基因为神经新生、细胞增殖和神经退行性改变富集的差异基因唯一交集,其mRNA表达量在Rbm8a基因表达恢复组出现下降。这些结果表明,恢复Rbm8a基因在齿状回的表达,不仅促进了神经新生,还减轻了AD疾病中的病理表型,这有助于促进AD认知功能的改善,其作用有可能是通过抑制Adora2a实现。

图1 Rbm8a基因的表达量随着野生型小鼠(WT)年龄增长逐渐下降,与WT小鼠相比,5xFAD小鼠(阿尔茨海默症的小鼠模型)的Rbm8a基因表达量更低。

图2 Rbm8a基因在海马齿状回过表达促进了5xFAD小鼠的新生神经元增多
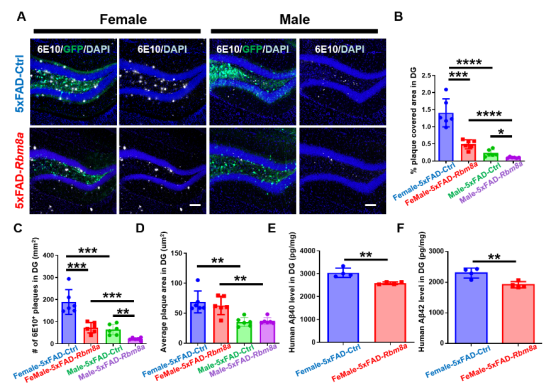
图3 Rbm8a基因过表达减少了5xFAD小鼠的老年斑块和营养不良神经突起的产生
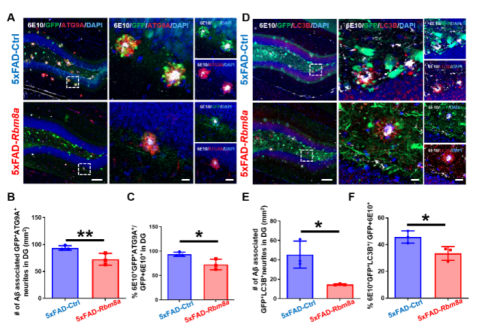
图4 Rbm8a基因过表达减少了5xFAD小鼠的老年斑块和营养不良神经突起的产生
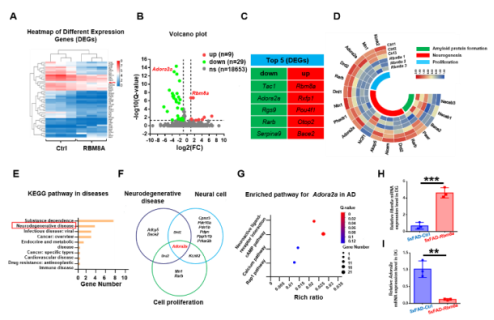
图5 RNA测序揭示Rbm8a促进神经新生和抑制斑块形成的可能性机制
总之,Rbm8a基因在调控神经新生中发挥着重要的作用。作者的研究表明,与正常小鼠相比,Rbm8a基因的表达在AD小鼠模型中明显下降。恢复Rbm8a基因的正常表达发现,齿状回的神经新生增加,病理改变减轻,从而有助于改善AD的认知功能。此项研究结果将为改善AD疾病中神经退行性改变提供新的治疗方向。
虽然文章提供了动物实验的证据,证实Rbm8a在促进神经发生、抑制斑块形成、增强认知功能等方面的积极作用,但也必须承认其局限性。首先,AAV2血清型病毒仅被特异性地注射到局部脑区以过表达Rbm8a,这并不能反映Rbm8a对含有干细胞的其他脑区更广泛的影响。其次,该研究仅观察了早期5xFAD小鼠,而Rbm8a在以干细胞数量较少为特征的晚期AD中的作用尚不清楚。第三,阿尔茨海默病涉及多种动物模型,但该研究利用5xFAD小鼠,而不同的AD模型具有各自的特点,这可能限制了研究结果的普遍性。最后,尽管Rbm8a过表达已被证明可促进神经干细胞增殖,但这种作用是否会诱导异常增殖并可能导致肿瘤发生尚不清楚。
原文链接:https://doi.org/10.4103/1673-5374.382254
参考文献
[1] Niklison-Chirou MV, Agostini M, Amelio I, et al. Regulation of Adult Neurogenesis in Mammalian Brain. Int J Mol Sci. 2020;21(14):4869.
[2] McSweeney C, Dong F, Chen M, et al. Full function of exon junction complex factor, Rbm8a, is critical for interneuron development. Transl Psychiatry. 2020;10(1):379.
[3] Zhang Y, Shen B, Zhang D, et al. miR-29a regulates the proliferation and differentiation of retinal progenitors by targeting Rbm8a. Oncotarget. 2017;8(19):31993-32008.
[4] Su CH, Liao WJ, Ke WC, et al. The Y14-p53 regulatory circuit in megakaryocyte differentiation and thrombocytopenia. iScience. 2021;24(11):103368.

通讯作者:王永刚教授






