中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (11): 3245-3258.doi: 10.4103/NRR.NRR-D-23-01144
人神经干细胞来源细胞外囊泡对缺血性脑卒中的神经保护
Human neural stem cell–derived extracellular vesicles protect against ischemic stroke by activating the PI3K/ AKT/mTOR pathway
Jiayi Wang1, 2, #, Mengke Zhao1, 2, #, Dong Fu2, 3, Meina Wang1, 2, Chao Han1, 2, Zhongyue Lv1, 2, Liang Wang1, 2, *, Jing Liu1, 2, *
- 1 Stem Cell Clinical Research Center, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province, China; 2 Dalian Innovation Institute of Stem Cell and Precision Medicine, Dalian, Liaoning Province, China; 3 Institute (College) of Integrative Medicine, Dalian Medical University, Dalian, Liaoning Province, China
摘要:
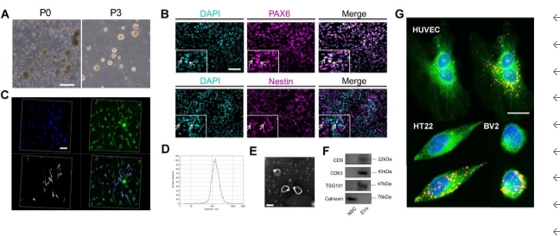
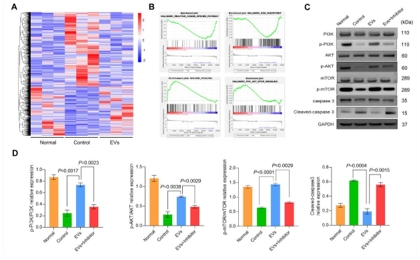
人神经干细胞来源细胞外囊泡为开发治疗缺血性脑卒中的有效方法打开了新思路;然而,其有效性、安全性及确切机制仍然需要进一步的研究。此临床前研究发现,缺血性脑卒中大鼠模型右侧壳核移植人神经干细胞来源细胞外囊泡后,脑梗死体积明显缩小,神经细胞凋亡明显减少,神经功能恢复,且具有良好的体内安全性。人神经干细胞来源细胞外囊泡通过激活磷酸肌酸3-激酶(PI3K)、哺乳动物雷帕霉素靶标(mTOR)和蛋白激酶B(AKT)的磷酸化表达减少了神经元凋亡,而PI3K抑制剂可逆转磷酸肌酸3-激酶的磷酸化表达。以上数据表明,人神经干细胞来源细胞外囊泡通过激活PI3K/AKT/mTOR信号通路对缺血性脑卒中发挥神经保护作用,其有望成为治疗缺血性脑卒中的潜在策略。
https://orcid.org/0000-0002-0493-296X (Jing Liu); https://orcid.org/0000-0003-2244-7535 (Liang Wang)