中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (8): 2408-2419.doi: 10.4103/NRR.NRR-D-23-01301
AAV2-PDE6B 抑制细胞凋亡拯救Rd10 模型小鼠视网膜色素变性
AAV2-PDE6B restores retinal structure and function in the retinal degeneration 10 mouse model of retinitis pigmentosa by promoting phototransduction and inhibiting apoptosis
Ruiqi Qiu1, 2, #, Mingzhu Yang1, 2, #, Xiuxiu Jin1, 2, 3, Jingyang Liu1, 2, Weiping Wang1, 2, Xiaoli Zhang1, 4, Jinfeng Han1, 4, Bo Lei1, 2, 3, 4, *
- 1 Henan Eye Hospital, Henan Provincial People’s Hospital, People’s Hospital of Zhengzhou University, Zhengzhou, Henan Province, China; 2 Eye Institute, Henan Academy of Innovations in Medical Science, Zhengzhou, Henan Province, China; 3 Branch of National Clinical Research Center for Ocular Disease, Henan Provincial People’s Hospital, Zhengzhou, Henan Province, China; 4 Academy of Medical Science, Zhengzhou University, Zhengzhou, Henan Province, China
摘要:
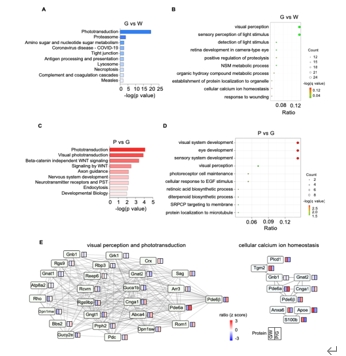
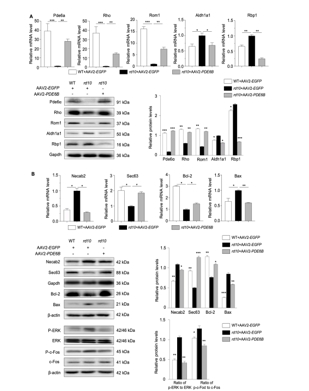
AAV是一种安全有效的基因运载工具,其介导的基因治疗已成为遗传性视网膜疾病的治疗的主流方向。动物实验证实AAV介导的PDE6B治疗能显著改善视网膜的结构与功能,然而AAV-PDE6B载体发挥治疗作用的具体分子机制以及分子水平上的变化尚不明确。为了解AAV2-PDE6B 改善视网膜色素变性视网膜功能的潜在分子机制。实验于rd10小鼠视网膜下注射AAV2-PDE6B后,使用暗适应和光适应视网膜电图、光学相干断层扫描和免疫荧光评估了对视网膜功能和结构的治疗效果。实验进一步进行了基于数据独立采集-质谱的蛋白质组分析,以研究蛋白质表达和通路富集,并通过实时 PCR 和 Western blot进一步筛选和验证。发现注射AAV2-PDE6B能显著提高PDE6β的水平,保护rd10小鼠的视网膜电图和核外层厚度。野生型小鼠和rd10小鼠的差异表达蛋白(DEPs)与视觉感知密切相关,AAV2-PDE6B治疗后视觉感知得到恢复。注射AAV2-PDE6B后,KEGG富集的最明显变化途径是光传导。此外,与光传导相关的蛋白 Pde6α,Rom1,Rho,Aldh1a1,Rbp1 表达也发生了逆转。rd10小鼠经AAV2-PDE6B处理后,Bax/Bcl-2,p-ERK/ERK和p-c-Fos/c-Fos水平均下降。实验数据表明,AAV2-PDE6B介导的基因治疗通过促进光传导和抑制 ERK1/2 介导的细胞凋亡发挥保护视网膜色素变性视网膜作用。