中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (10): 2285-2290.doi: 10.4103/1673-5374.369124
由Notch信号通路介导的星形胶质细胞-神经元通讯:关注谷氨酸转运和突触可塑性
Astrocyte-neuron communication mediated by the Notch signaling pathway: focusing on glutamate transport and synaptic plasticity
Ke-Xin Li, Meng Lu, Meng-Xu Cui, Xiao-Ming Wang*, Yang Zheng*
- Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, Liaoning Province, China
摘要:
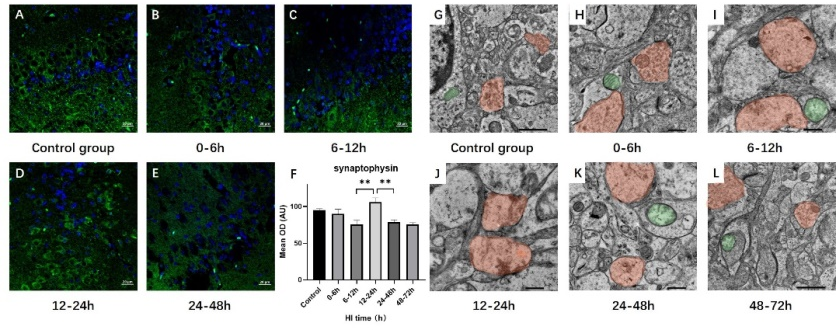
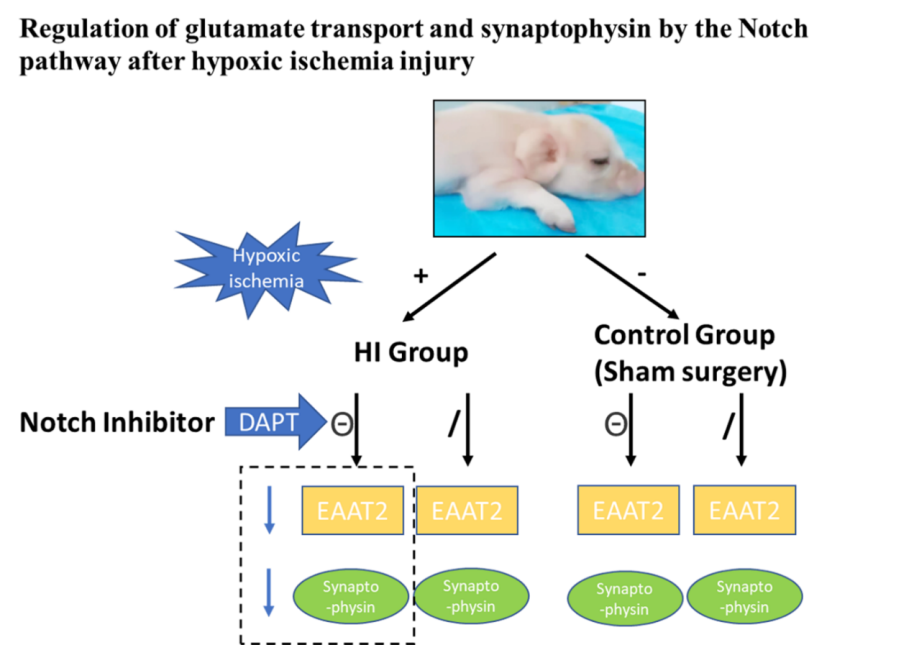
维持神经递质的平衡是星形胶质细胞对神经元的支持作用之一。其中,谷氨酸的平衡是维持突触功能和神经细胞活动的一个重要方面。然而,研究谷氨酸的动态变化、谷氨酸转运的分子机制以及对突触的影响尚需进一步研究。为探讨Notch信号通路介导的谷氨酸转运和突触可塑性的调节机制,实验以暂时性阻断双侧颈总动脉40min,同时机械吸入6%的氧气的方法来制作新生猪急性缺氧缺血性脑病模型。建模后,随机将动物分为Notch通路抑制组及Notch通路非抑制组,Notch通路抑制组动物以3.33 mg/mL的浓度腹腔注射Notch通路抑制剂(DAPT),非抑制组动物注射等剂量DMSO。应用免疫组化、免疫荧光及免疫印迹实验评估脑内蛋白表达情况。应用透射电镜观察脑内微观结构变化。在缺氧缺血性脑损伤早期(损伤后6-12h),谷氨酸转运体兴奋性氨基酸转运体2和突触蛋白下调,突触小泡数量减少,突触肿胀;在损伤后12-24h,Notch通路被激活,谷氨酸转运体兴奋性氨基酸转运体2和突触蛋白的表达增加,突触小泡数量略有增加。反过来,在应用Notch途径抑制剂后,谷氨酸转运体和突触蛋白的表达下降。这表明,缺氧缺血性脑损伤后星形胶质细胞-神经元的谷氨酸转运受Notch途径调节,并通过突触蛋白的表达影响囊泡释放和突触可塑性。
https://orcid.org/0000-0002-7452-0725 (Yang Zheng); https://orcid.org/ 0000-0003-0276-2466 (Xiao-Ming Wang)