NRR:解放军北部战区总医院陈会生团队提出Vav1沉默可改善脑缺血再灌注损伤
撰文:仇靖、郭俊、刘亮、刘欣、孙显辉、陈会生
脑缺血是世界范围内脑部残障的主要原因,也是仅次于心血管疾病和癌症的第三大死因[1]。临床上治疗脑缺血的原则之一是尽早恢复血液再灌注,然而脑缺血再灌注也可引起脑损伤,导致脑水肿、脑出血和神经死亡[2]。脑缺血再灌注损伤的病理机制十分复杂。有研究表明,免疫反应引起的脑缺血后炎症是导致脑缺血再灌注损伤的重要因素[3],而小胶质细胞作为中枢神经系统的巨噬细胞在炎症反应和抗脑缺血中具有重要的意义[4]。Vav1(Vav Guanine Nucleotide Exchange Factor 1,也称为Vav)是一种鸟苷酸激活因子,在多种中枢神经系统疾病中的神经元凋亡及炎症因子产生中发挥着重要的调节作用[5, 6]。大量研究表明,Vav1表达可能与小胶质细胞激活相关[7],这提示Vav1可能与神经炎症密切相关。但Vav1在小胶质细胞激活中发挥着怎么样的作用,以及其如何通过调控小胶质细胞活动以改善脑缺血再灌注损伤后炎症反应,尚不明确。
来自中国人民解放军北部战区总医院陈会生团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Vav1 promotes inflammation and neuronal apoptosis in cerebral ischemia/reperfusion injury by upregulating microglial and NLRP3 inflammasome activation”的研究发现,Vav1沉默可显著缩小大脑中动脉闭塞再灌注大鼠的脑梗死面积,并减轻其神经元损伤及凋亡。进一步地研究显示,Vav1主要存在于小胶质细胞,其沉默可抑制小胶质细胞激活,并通过抑制NLRP3介导的炎症因子释放,减轻炎症反应。该研究揭示了Vav1可通过NLRP3介导的炎症反应,促进神经元损伤和凋亡,进而加剧脑缺血再灌注损伤。这可能为开发脑缺血再灌注损伤的药物提供帮助。
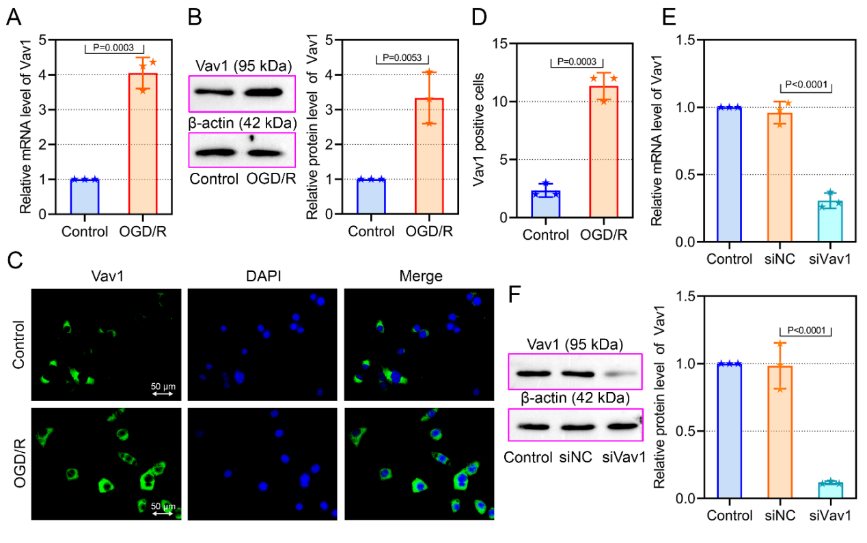
Vav1已被证明在多种中枢神经系统疾病神经元凋亡及炎症因子的产生中发挥重要调节作用[5, 6],且陈会生等已通过GEO数据库(GSE163614)发现Vav1在大脑中动脉闭塞再灌注大鼠中呈现高表达。因此,此次实验首先探究了Vav1在大脑中动脉闭塞再灌注大鼠中mRNA及蛋白水平,结果证实其表达上调(图1),而敲除Vav1可减轻大脑中动脉闭塞再灌注大鼠的脑梗死提及和神经元损伤(图2)。这表明Vav1表达与脑缺血再灌注损伤程度密切相关。此外,既往 研究证实了脑缺血再灌注损伤可导致神经元变性和凋亡,而下调Vav1表达可减少神经元凋亡[5],此次研究结果与之相符,即敲低Vav1可减轻大脑中动脉闭塞再灌注大鼠的神经元损失和凋亡(图3)。综上提示,Vav1敲低对神经元损伤有保护作用,因此降低其表达可能是改善脑缺血再灌注损伤的关键策略。
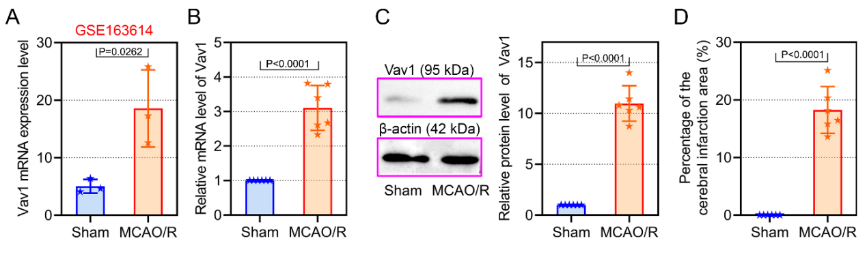
图1大脑中动脉闭塞再灌注大鼠Vav1表达上调(图源:Qiu et al., Neural Regen Res, 2023)

图2 敲低Vav1可减轻大脑中动脉闭塞再灌注大鼠的脑梗死体积和神经元损伤(图源:Qiu et al., Neural Regen Res, 2023)
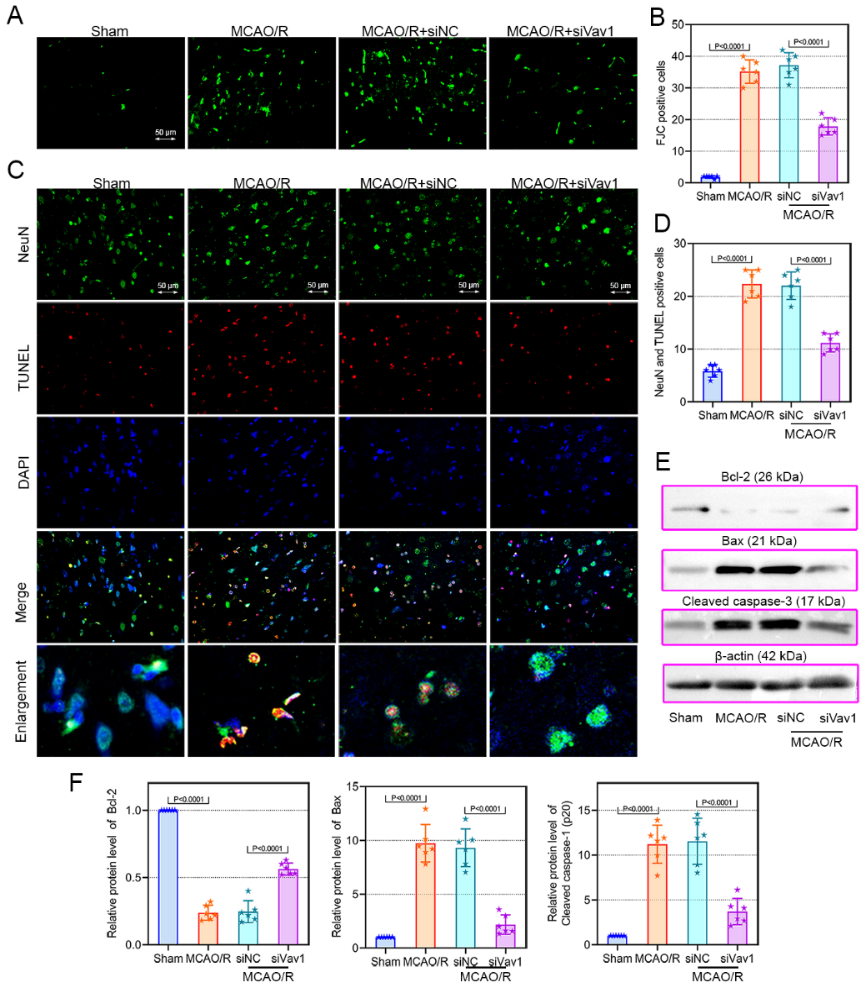
图3 敲低Vav1可减轻大脑中动脉闭塞再灌注大鼠的神经元损失和凋亡(图源:Qiu et al., Neural Regen Res, 2023)
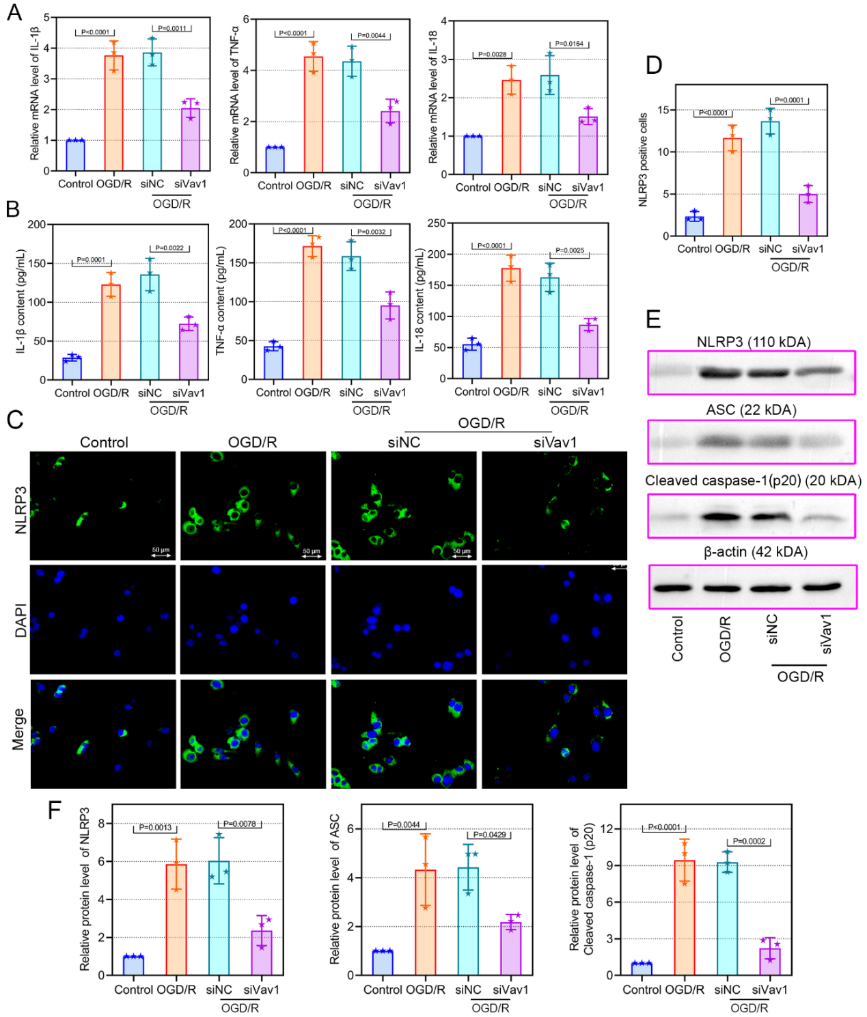
免疫反应引起的脑缺血后炎症是导致脑缺血再灌注损伤的重要因素[3],而小胶质细胞作为中枢神经系统的巨噬细胞,可参与炎症反应,并在缺血后早期被激活[4]。此外,小胶质细胞产生的促炎因子,如白细胞介素1β、白细胞介素6、肿瘤坏死因子α及活性氧是脑缺血神经元死亡的重要因素[8]。因此抑制小胶质细胞的激活及其介导的炎症反应可能是神经实质抵抗脑缺血的关键。因此,敲低Vav1对大脑中动脉闭塞再灌注大鼠中小胶质细胞和炎症小体的激活的作用。此次实验验证了Vav1的表达与小胶质细胞激活相关的结论[7],且发现敲低Vav1可抑制大脑中动脉闭塞再灌注大鼠小胶质细胞和NLRP3炎性小体激活(图4),并抑制其炎症反应(图5)。以上结果表明,Vav1敲低可能通过抑制小胶质细胞和NLRP3炎症小体的激活,减轻脑缺血再灌注大鼠的炎症反应和神经元凋亡。
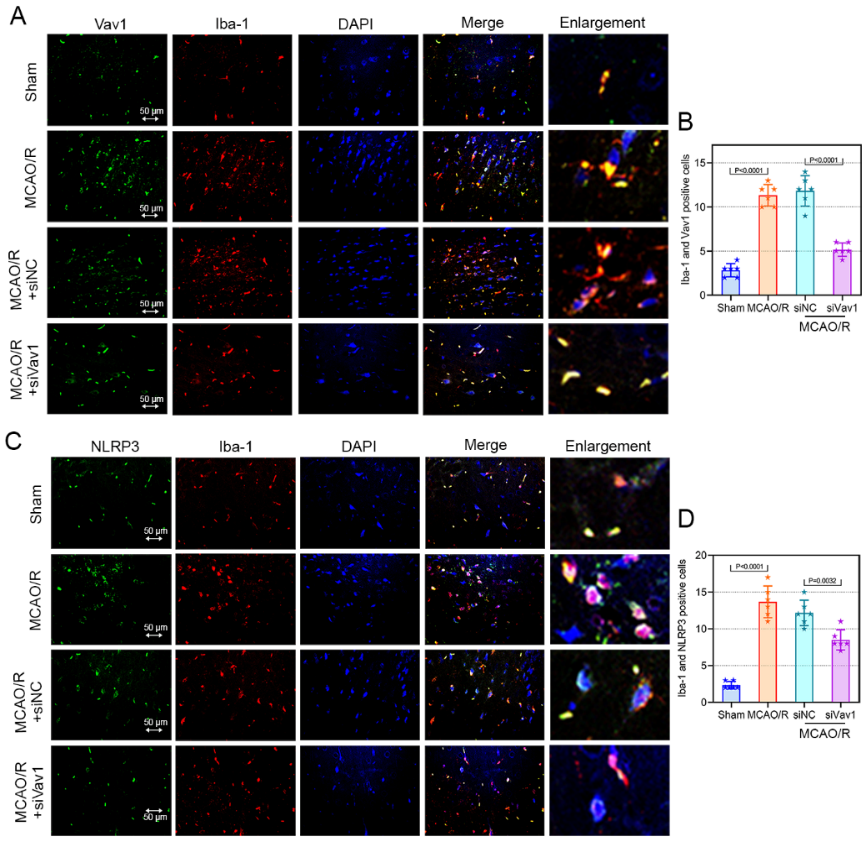
图4敲低Vav1可抑制大脑中动脉闭塞再灌注大鼠小胶质细胞和NLRP3炎性小体激活(图源:Qiu et al., Neural Regen Res, 2023)
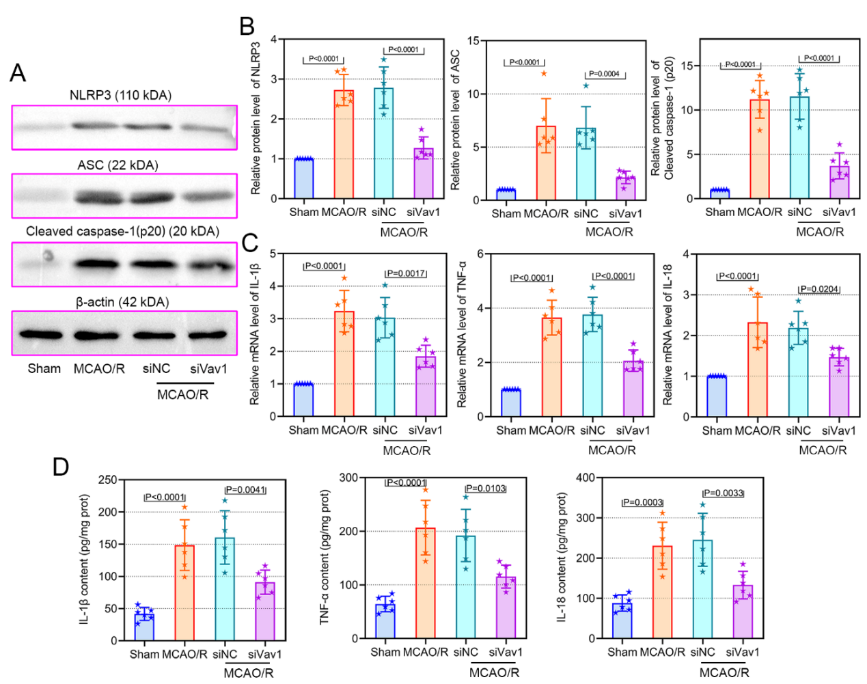
图5 敲低Vav1可抑制大脑中动脉闭塞再灌注大鼠的炎症反应(图源:Qiu et al., Neural Regen Res, 2023)
脑缺血再灌注损伤病理机制复杂,目前公认的理论包括炎症、氧化应激凋亡以及坏死等[2],有证据表明脑缺血后的炎症反应是导致脑缺血再灌注损伤的重要因素[3]。实验进一步在体外验证以上结论,在氧糖剥夺复氧诱导小鼠小胶质细胞BV-2细胞中评估了Vav1表达与小胶质细胞介导的炎症反应的关系。上述体内实验结果一致,在氧糖剥夺复氧后,BV-2细胞中Vav1水平升高(图6),而敲低Vav1可显著抑制其炎症反应(图7)。
 #br#
#br#
图6氧糖剥夺复氧诱导的BV-2细胞中Vav1表达增加(图源:Qiu et al., Neural Regen Res, 2023)
 #br#
#br#
图7敲低Vav1可抑制氧糖剥夺复氧的BV-2细胞中的炎症反应(图源:Qiu et al., Neural Regen Res, 2023)
该研究于体内外水平上证实了Vav1沉默可通过抑制小胶质细胞和NLRP3炎症小体激活,减轻脑缺血再灌注损伤引起的炎症反应和神经元凋亡。但是由于体内实验中选择在造模前下调Vav1的表达,尚不能充分体现其治疗价值,因此需考虑在术后敲低Vav1表达是否也能得到相似的结果。此外,该研究未涉及临床样本,因此仍需进行进一步临床转化的研究。
原文链接:https://doi.org/10.4103/1673-5374.371368
参考文献
[1] Stegner D, Klaus V, Nieswandt B. Platelets as modulators of cerebral ischemia/reperfusion injury. Front Immunol. 2019;10:2505.
[2] Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181-198.
[3] Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32(9):1677-1698.
[4] Takeda H, Yamaguchi T, Yano H, et al. Microglial metabolic disturbances and neuroinflammation in cerebral infarction. J Pharmacol Sci. 2021;145(1):130-139.
[5] He B, Chen W, Zeng J, et al. MicroRNA-326 decreases tau phosphorylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer's disease. J Cell Physiol. 2020;235(1):480-493.
[6] Hong J, Min Y, Wuest T, et al. Vav1 is essential for HIF-1α activation via a lysosomal VEGFR1-mediated degradation mechanism in endothelial cells. Cancers (Basel). 2020;12(6):1374.
[7] Chen Y, Jin Y, Zhan H, et al. Proteomic analysis of the effects of Nur77 on lipopolysaccharide-induced microglial activation. Neurosci Lett. 2017;659:33-43.
[8] Surinkaew P, Sawaddiruk P, Apaijai N, et al. Role of microglia under cardiac and cerebral ischemia/reperfusion (I/R) injury. Metab Brain Dis. 2018;33(4):1019-1030.





 #br#
#br#
 #br#
#br#