NRR:深圳市中医院卜凡团队揭示缺血性血脑屏障新作用机制
撰文:卜凡
血脑屏障可选择性隔绝脑与外周环境,维持脑内代谢平衡。缺血性脑卒中能够引起血脑屏障损伤,造成脑水肿、脑出血和外周免疫细胞侵入,这是脑损伤加重的重要原因之一[1]。已知内皮细胞的紧密连接蛋白表达分布异常可增加细胞间隙和血脑屏障通透性,但其上游调控机制尚未完全阐明。丝裂原活化蛋白激酶磷酸酶1(mitogen-activated protein kinase phosphatase 1,MKP-1)是丝裂原活化蛋白激酶家族多个亚型的负调控蛋白。既往研究显示,选择性抑制丝裂原活化蛋白激酶能够缓解多种外界刺激(如盐摄入过量[2]和缺氧预处理[3]等)引起的内皮细胞紧密连接功能表达失调。敲除丝裂原活化蛋白激酶磷酸酶1能够加重小鼠缺血性脑损伤[4],然而其是否影响血脑屏障完整性和细胞特异性机制尚不明确。
最近中国深圳市中医院卜凡团队和嘉兴市第一医院徐龙生团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“Overexpression of mitogen-activated protein kinase phosphatase-1 in endothelial cells reduces blood-brain barrier injury in a mouse model of ischemic stroke”的研究。结果发现,过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1可显著缓解脑缺血引起的血脑屏障损伤,同时促进神经功能恢复。而丝裂原活化蛋白激酶磷酸酶1负调控丝裂原活化蛋白激酶/细胞外调节蛋白激酶1/2对Occludin蛋白表达的抑制作用是其保护血脑屏障完整性的分子基础。这项研究从整体、细胞和分子水平上丰富了对缺血性血脑屏障损伤机制的认知。
血脑屏障是主要由脑毛细血管壁与神经胶质细胞形成的选择性隔绝脑与外周环境,维持脑内代谢平衡的屏障。选择性抑制丝裂原活化蛋白激酶多种亚型能够缓解盐摄入过量和缺氧预处理等外界刺激引起的内皮细胞紧密连接功能表达下调[2, 3]。敲除丝裂原活化蛋白激酶磷酸酶1能够加重小鼠缺血性脑损伤和神经功能缺失[4]。因此卜凡等猜测丝裂原活化蛋白激酶磷酸酶1可能通过负调控丝裂原活化蛋白激酶活性的方式,阻止脑缺血引起的内皮细胞紧密连接表达异常,进而缓解血脑屏障渗漏和脑损伤。
此项研究通过脑实质内注射慢病毒的方式过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1,然后利用行为学、组织化学、免疫荧光和蛋白印迹等手段检测暂时性脑缺血小鼠感觉运动和记忆功能、缺血梗死灶体积和微血管完整性等的变化。然后利用原代内皮细胞建立细胞单层屏障,予以氧糖剥夺刺激,并药理学抑制丝裂原活化蛋白激酶磷酸酶1和其负调控底物细胞外调节蛋白激酶1/2的活性,在体外缺血模型上进一步论证丝裂原活化蛋白激酶磷酸酶1/细胞外调节蛋白激酶1/2通路对内皮细胞损伤、紧密连接表达以及皮屏障完整性的调节机制。
行为学结果首先发现,损伤前过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1能显著缓解暂时性脑缺血(1h大脑中动脉阻塞)引起的整体神经功能缺失,并促进感觉运动和认知记忆功能的恢复(图1)。
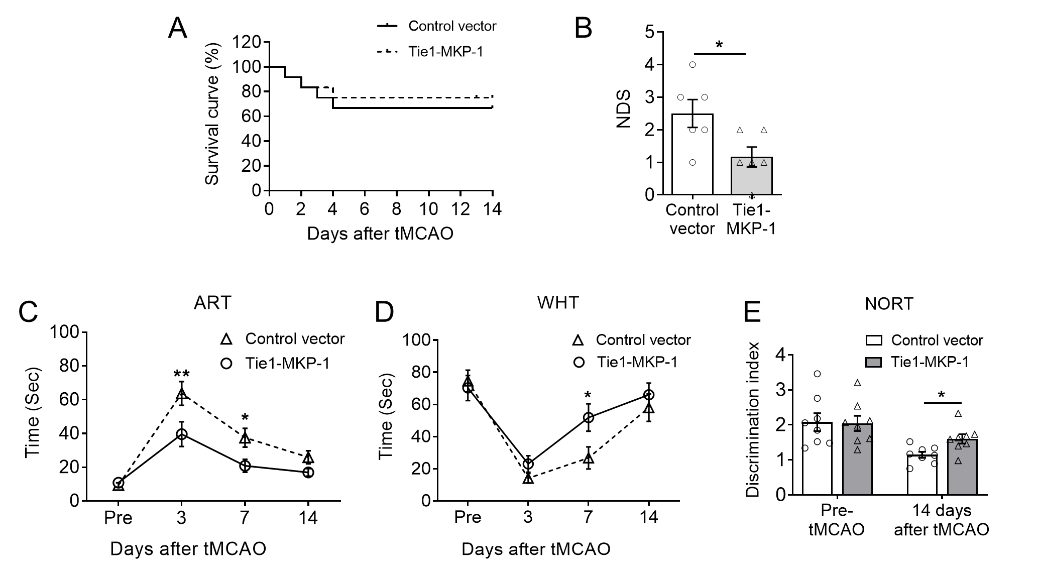
图1. 小鼠脑内过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1对脑缺血后感觉运动和记忆认知行为的作用。(图源:Qin et al., Neural Regen Res, 2023)。
然后在脑缺血后第3天尾静脉给予植物血凝素标记血管,荧光成像发现过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1可显著阻止暂时性脑缺血引起的血凝素信号强度减弱,还能阻止缺血半暗带中同源IgG免疫荧光信号的升高。提示过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1能够缓解脑缺血引起的血脑屏障损伤(图2A和B)。由于外周免疫细胞能够透过受损血脑屏障侵入大脑,增加缺血急性期的脑炎性损伤。因此实验还采用酶联免疫吸附法测定脑实质多种炎症因子的含量,可见过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1能够逆转暂时性脑缺血后第3天时白细胞介素1β、白细胞介素6和趋化因子配体2的升高,这进一步证实了内皮细胞丝裂原活化蛋白激酶磷酸酶1对缺血性脑损伤的保护作用(图2C)。
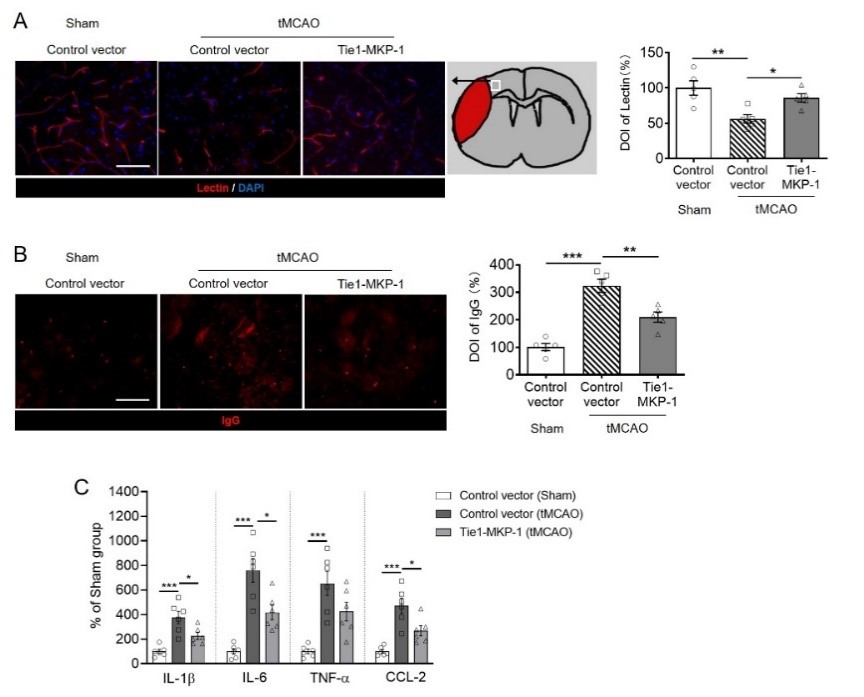
图2. 小鼠脑内过表达内皮细胞丝裂原活化蛋白激酶磷酸酶1对脑缺血后血脑屏障完整性和脑内炎症因子含量的影响。(图源:Qin et al., Neural Regen Res, 2023)。
进而利用体外模型探索丝裂原活化蛋白激酶磷酸酶1调节血脑屏障完整性的分子机制,在半透性筛网上培养原代内皮细胞构建单层屏障,并给予氧糖剥夺刺激。结果可见,药理学抑制丝裂原活化蛋白激酶磷酸酶1能加重氧糖剥夺引起的细胞屏障渗漏和紧密连接 occludin表达下调;而抑制丝裂原活化蛋白激酶磷酸酶1负调节底物丝裂原活化蛋白激酶/细胞外调节蛋白激酶1/2则可起到相反的作用。此外,同时给予丝裂原活化蛋白激酶磷酸酶1和细胞外调节蛋白激酶1/2的抑制剂并不能改变单独给予细胞外调节蛋白激酶1/2的保护效果(图3),表明丝裂原活化蛋白激酶磷酸酶1能通过负调节细胞外调节蛋白激酶1/2对Occludin的表达抑制,阻止屏障损伤。
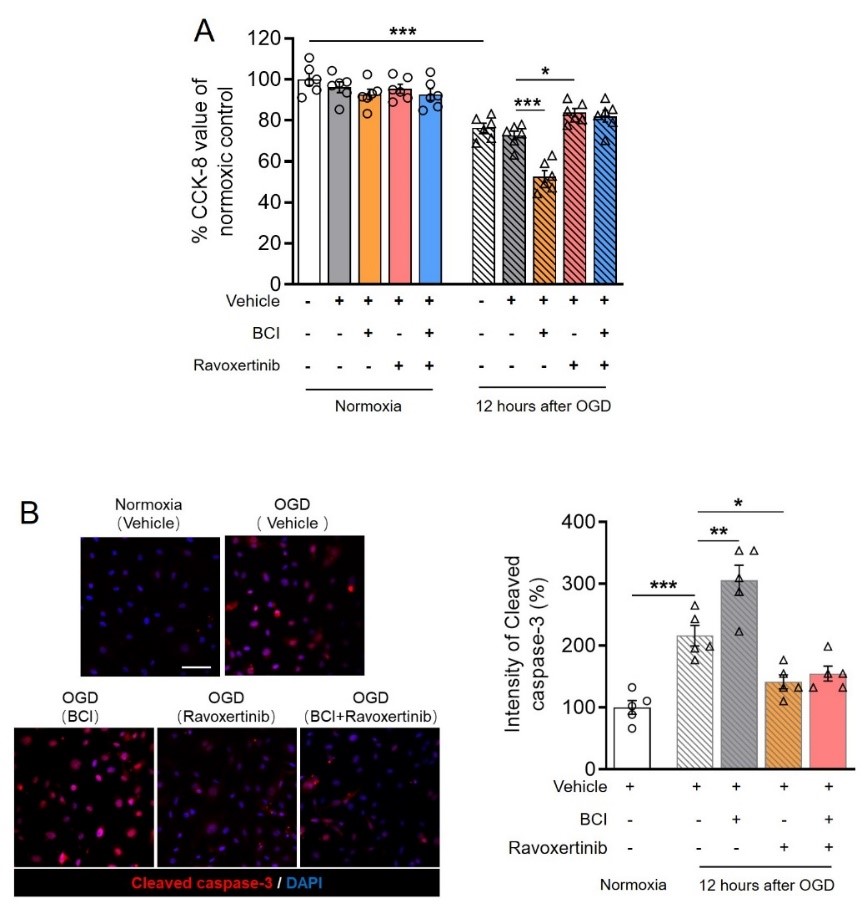
图3. 药理学抑制丝裂原活化蛋白激酶磷酸酶1和/或细胞外调节蛋白激酶1/2对离体内皮细胞屏障和occludin表达的影响。(图源:Qin et al., Neural Regen Res, 2023)。
最后在细胞模型上利用药理学手段检测丝裂原活化蛋白激酶磷酸酶1/细胞外调节蛋白激酶1/2通路对内皮细胞活性的影响。发现抑制丝裂原活化蛋白激酶磷酸酶1可进一步降低氧糖剥夺后细胞活性,并加剧细胞死亡;而细胞外调节蛋白激酶1/2抑制剂起到相反的作用。同时抑制丝裂原活化蛋白激酶磷酸酶1和细胞外调节蛋白激酶1/2与单独给予细胞外调节蛋白激酶1/2抑制剂无显著差异(图4),表明丝裂原活化蛋白激酶磷酸酶1/细胞外调节蛋白激酶1/2通路亦参与调节缺血性内皮细胞死亡。
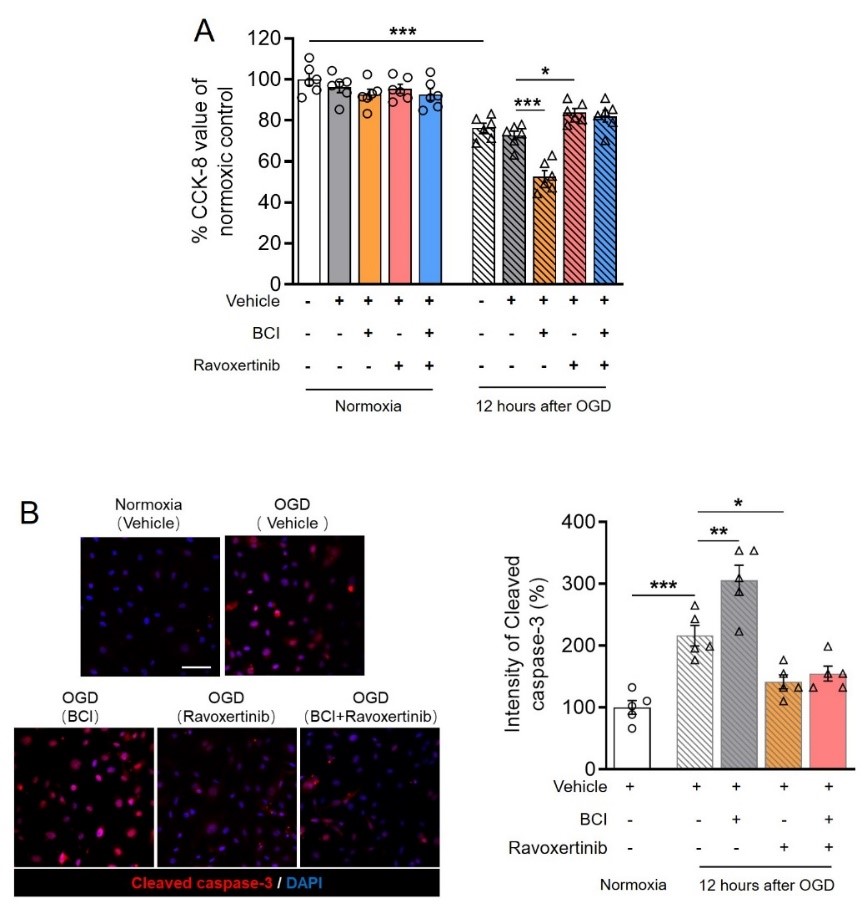
图4. 药理学抑制丝裂原活化蛋白激酶磷酸酶1和/或细胞外调节蛋白激酶1/2对离体内皮细胞活性的影响。(图源:Qin et al., Neural Regen Res, 2023)。
综上表明,脑缺血条件下激活脑内皮细胞丝裂原活化蛋白激酶磷酸酶1能够负调节细胞外调节蛋白激酶1/2对紧密连接以及occludin表达的抑制作用,进而保护血脑屏障完整性,减轻脑损伤并促进脑卒中预后。
该研究基于动物和细胞模型揭示了内皮细胞丝裂原活化蛋白激酶磷酸酶1在缺血性血脑屏障损伤中的调节作用,并深入解析了其通过负调控细胞外调节蛋白激酶1/2对occludin表达的抑制作用的分子机制。特异性激活内皮细胞丝裂原活化蛋白激酶磷酸酶1可能是治疗缺血性脑损伤的新策略。然而,脑缺血是一种老年性疾病,且存在性别差异,如老年女性脑卒中预后显著劣于男性。由于研究仅在成年雄性小鼠上确定了内皮细胞丝裂原活化蛋白激酶磷酸酶1的作用机制,尚不能确定其在缺血性血脑屏障损伤中的保护作用是否存在年龄和性别差异。此外实验在脑卒中前进行过表达丝裂原活化蛋白激酶磷酸酶1干预,但在脑卒中后进行干预是否仍具有治疗效果目前亦不清楚。因此为来,需要在不同性别模型以及干预时间时探索缺血性脑卒中后干预内皮细胞丝裂原活化蛋白激酶磷酸酶1对脑损伤的治疗效果,以期为研究结果的临床转化奠定更为坚实的基础。
原文链接:https://doi.org/10.4103/1673-5374.363836#br#
#br#
参考文献#br#
[1] Jiang X, Andjelkovic AV, Zhu L, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164:144-171.#br#
[2] Zhang T, Fang S, Wan C, et al. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci Rep. 2015;5:16548.#br#
[3] Shin JA, Kim YA, Jeong SI, et al. Extracellular signal-regulated kinase1/2-dependent changes in tight junctions after ischemic preconditioning contributes to tolerance induction after ischemic stroke. Brain Struct Funct. 2015;220(1):13-26.#br#
[4] Liu L, Doran S, Xu Y, et al. Inhibition of mitogen-activated protein kinase phosphatase-1 (MKP-1) increases experimental stroke injury. Exp Neurol. 2014;261:404-411.#br#
[5] González-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778(3):729-756.#br#
#br#
第一作者:秦秀德,深圳市中医院,脑病与心理病科主任,主任医师。#br#
基金支持:深圳市医疗卫生三名工程(SZZYSM202111011)。
 #br#
通讯作者:卜凡,深圳市中医院,副研究员,2017-2020年在美国得克萨斯大学休斯顿健康科学中心接受博士后训练,曾获美国心脏协会博士后研究基金资助,研究兴趣为缺血性脑损伤的分子机制和干预。#br#
基金支持:深圳市医疗卫生三名工程(SZZYSM202111011),深圳市中医院引进专职科研人才项目启动经费(No.2021-07)。#br#
#br#
#br#
通讯作者:徐龙生,嘉兴市第一医院,副研究员,中心实验室主任。#br#
基金支持:嘉兴市省市共建医学重点学科建设计划 (2019-ss-ttyx),嘉兴市神经与疼痛医学重点实验室(嘉科综【2014】81号)。
#br#
通讯作者:卜凡,深圳市中医院,副研究员,2017-2020年在美国得克萨斯大学休斯顿健康科学中心接受博士后训练,曾获美国心脏协会博士后研究基金资助,研究兴趣为缺血性脑损伤的分子机制和干预。#br#
基金支持:深圳市医疗卫生三名工程(SZZYSM202111011),深圳市中医院引进专职科研人才项目启动经费(No.2021-07)。#br#
#br#
#br#
通讯作者:徐龙生,嘉兴市第一医院,副研究员,中心实验室主任。#br#
基金支持:嘉兴市省市共建医学重点学科建设计划 (2019-ss-ttyx),嘉兴市神经与疼痛医学重点实验室(嘉科综【2014】81号)。




 #br#
通讯作者:卜凡,深圳市中医院,副研究员,2017-2020年在美国得克萨斯大学休斯顿健康科学中心接受博士后训练,曾获美国心脏协会博士后研究基金资助,研究兴趣为缺血性脑损伤的分子机制和干预。#br#
基金支持:深圳市医疗卫生三名工程(SZZYSM202111011),深圳市中医院引进专职科研人才项目启动经费(No.2021-07)。#br#
#br#
#br#
通讯作者:徐龙生,嘉兴市第一医院,副研究员,中心实验室主任。#br#
基金支持:嘉兴市省市共建医学重点学科建设计划 (2019-ss-ttyx),嘉兴市神经与疼痛医学重点实验室(嘉科综【2014】81号)。
#br#
通讯作者:卜凡,深圳市中医院,副研究员,2017-2020年在美国得克萨斯大学休斯顿健康科学中心接受博士后训练,曾获美国心脏协会博士后研究基金资助,研究兴趣为缺血性脑损伤的分子机制和干预。#br#
基金支持:深圳市医疗卫生三名工程(SZZYSM202111011),深圳市中医院引进专职科研人才项目启动经费(No.2021-07)。#br#
#br#
#br#
通讯作者:徐龙生,嘉兴市第一医院,副研究员,中心实验室主任。#br#
基金支持:嘉兴市省市共建医学重点学科建设计划 (2019-ss-ttyx),嘉兴市神经与疼痛医学重点实验室(嘉科综【2014】81号)。