NRR:山东大学王贞和青岛市立医院李彤联合团队提出硫化氢在亨廷顿病氧化应激中的新作用机制
撰文:王贞,李彤,江子格,刘德祥
亨廷顿病的发展与体内较高水平的神经毒素喹啉酸有关。喹啉酸可在体内累积导致氧化应激,从而引起神经毒性 。然而,喹啉酸在亨廷顿病病理生理进程中的分子和细胞机制尚不清楚。硫化氢(H2S)是内源性神经调节剂之一,主要由胱硫氨酸γ-裂解酶、胱硫氨酸β-合成酶和3-巯基丙酮酸硫转移酶三种酶组成。生理浓度下的H2S具有神经保护作用。有研究指出,H2S的保护作用可能与核因子e2相关因子2 (nuclear factor erythroid 2-related factor 2, Nrf2)密切相关 。因此,探讨内源性H2S对喹啉酸诱导的氧化应激的影响,并探讨胱硫氨酸β-合成酶/H2S通过Nrf2调节氧化应激的可能机制是非常必要的。
最近,来自中国山东大学王贞和青岛市立医院李彤联合团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Hydrogen sulfide reduces oxidative
stress in Huntington’s disease via Nrf2”的研究。文章发现给予小鼠纹状体和神经元喹啉酸后,其血浆和神经元中的H2S水平降低,同时伴有H2S合成酶胱硫氨酸β-合成酶的表达下调。而H2S供体NaHS模拟内源性H2S增加可缓解上述变化,并改善喹啉酸处理后的纹状体和神经元氧化失衡和线粒体功能障碍等一系列现象。H2S的这些有益作用与Nrf2表达的上调密切相关。而后以ML385抑制Nrf2的表达,则可逆转H2S对喹啉酸诱导的氧化应激的有益影响。该研究更全面地了解了内源性H2S在调节氧化还原稳态和减轻喹啉酸诱导的氧化应激中的有益作用,从而可能为治疗亨廷顿病开发新型神经保护药物提供方向。
纹状体内注射喹啉酸后,血浆中的H2S水平显著降低,这表明喹啉酸可导致小鼠内源性H2S生成减少,而NaHS则能上调H2S水平。在PC12细胞中也发现了类似的现象。接下来,王贞和李彤等又检测了喹啉酸对内源性H2S的重要合成酶胱硫氨酸β-合成酶表达的影响。结果显示,喹啉酸显著降低了小鼠纹状体以及PC12细胞中胱硫氨酸β-合成酶的表达,NaHS则能恢复其表达。研究还观察到在ML385可逆转NaHS诱导的高H2S水平以及胱硫氨酸β-合成酶表达(图1)。在给予喹啉酸后,小鼠运动功能出现明显损伤。NaHS能恢复喹啉酸诱导的小鼠运动功能障碍,而ML385则能逆转NaHS 的作用(图2)。综上,NaHS能改善喹啉酸导致的运动功能障碍,减轻氧化应激和细胞凋亡,同时对神经突触损伤以及线粒体功能障碍也发挥有益作用(图3)。
图1在小鼠纹状体和神经元中,NaHS处理后H2S水平升高以及胱硫氨酸β-合成酶表达增强(图源:Jiang et al., Neural Regen Res, 2025)
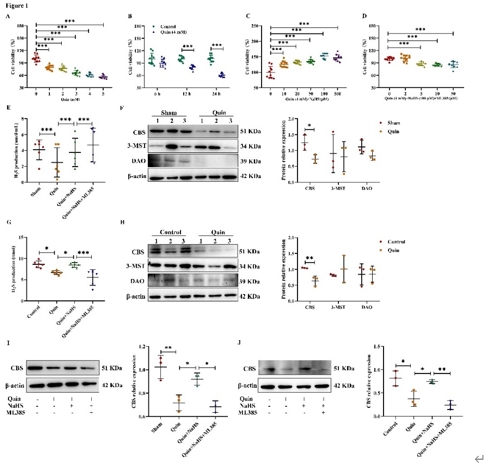
图2 NaHS改善喹啉酸诱导的神经行为损伤(图源:Jiang et
al., Neural Regen Res, 2025)
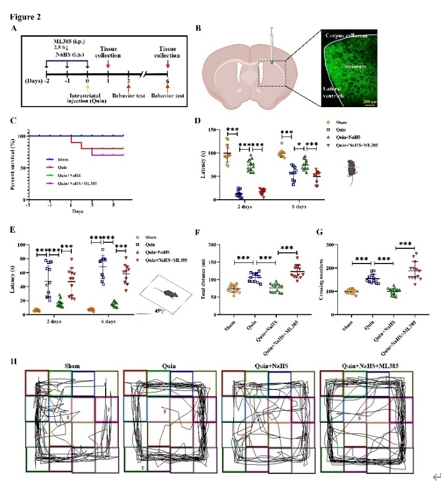
图3胱硫氨酸β-合成酶/H2S通过调节Nrf2,改善喹啉酸诱导的氧化应激(图源:Jiang et al., Neural Regen Res, 2025)
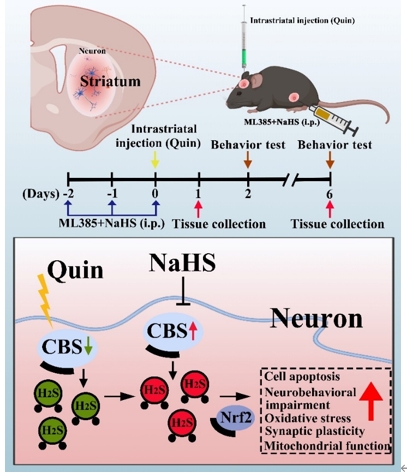
综上所述,王贞和李彤等证实了H2S在喹啉酸诱导的小鼠纹状体和神经元氧化应激中发挥作用。胱硫氨酸β-合成酶/H2S可通过激活Nrf2减轻氧化应激。该研究有助于加深对H2S参与喹啉酸诱导的氧化应激的潜在信号机制的理解,从而为开发神经保护药物提供新的靶点和指导。同时研究也存在一定的局限性。首先,只关注了主要的H2S生成酶胱硫氨酸β-合成酶在喹啉酸诱导的氧化应激中的作用,但实验中也发现其他的H2S生成酶,如D-氨基酸氧化酶,在小鼠纹状体中也有一定程度的减少,但在神经元中没有发现该现象。D-氨基酸氧化酶在喹啉酸诱导的氧化应激中的确切作用需要进一步研究。其次,胱硫氨酸β-合成酶/H2S激活Nrf2/血红素加氧酶1系统的机制仍不完全清楚,需要进一步研究。第三,体外实验主要基于PC12细胞系,需要进一步利用原代神经元来阐明H2S在喹啉酸诱导的氧化应激中的作用。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01051
参考文献
ADDIN EN.REFLIST [1] Beaumont V, Mrzljak L, Dijkman U, et
al. The novel KMO inhibitor CHDI-340246 leads to a restoration of
electrophysiological alterations in mouse models of Huntington's disease. Exp
Neurol. 2016;282:99-118.
[2] Martínez-Gopar PE, Pérez-Rodríguez MJ,
Rodríguez-Manzo G, et al. Mast cells and histamine are involved in the neuronal
damage observed in a quinolinic acid-induced model of Huntington's disease. J
Neurochem. 2022;160(2):256-270.
[3] Kubicova L, Hadacek F, Chobot V.
Quinolinic acid: neurotoxin or oxidative stress modulator? Int J Mol Sci.
2013;14(11):21328-21338.
[4] Kimura H. Hydrogen sulfide and
polysulfides as biological mediators. Molecules. 2014;19(10):16146-16157.
[5] Paul BD, Snyder SH. Gasotransmitter
hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol.
2018;149:101-109.
[6] Szabo C, Papapetropoulos A.
International union of basic and clinical pharmacology. CII: Pharmacological
modulation of H(2)S levels: H(2)S donors and H(2)S biosynthesis inhibitors.
Pharmacol Rev. 2017;69(4):497-564.
[7] Corsello T, Komaravelli N, Casola A.
Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular
redox balance. Antioxidants (Basel). 2018;7(10):129.


