中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (8): 2153-2168.doi: 10.4103/NRR.NRR-D-24-00088
创伤性脑损伤后脑-肠-微生物轴的双向调节
Bidirectional regulation of the brain–gut–microbiota axis following traumatic brain injury
Xinyu You1, 2, #, Lin Niu1, 2, #, Jiafeng Fu1, 2, Shining Ge1, 2, Jiangwei Shi3, 4, Yanjun Zhang3, 4, *, Pengwei Zhuang2, 3, *
- 1 National Key Laboratory of Chinese Medicine Modernization, Tianjin University of Traditional Chinese Medicine, Tianjin, China; 2 Haihe Laboratory of Modern Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China; 3 First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China; 4 National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
摘要:
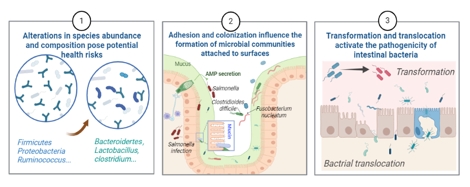
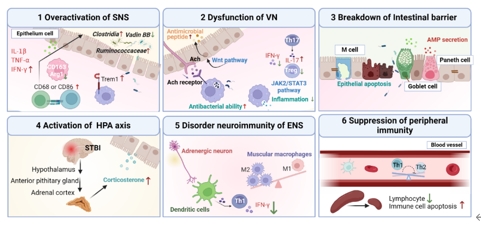
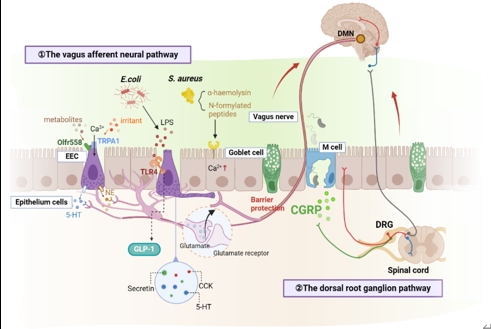
创伤性脑损伤是一种常见的中枢神经系统疾病,除原发性脑实质损伤外,其造成的持久生物学后果也是患者的长期风险。目前,创伤性脑损伤的发病机制仍不完全清楚,尚缺乏有效的干预方法。肠道功能障碍是创伤性脑损伤的一个重要后果,其作为体内受神经支配最丰富的外周组织,拥有多种途径与中枢神经系统形成双向“脑肠轴”。此外,肠道拥有庞大的微生物群落。肠道生态位的改变通过神经元、激素、免疫的传入和传出通路参与创伤性脑损伤及其不良预后的进展。因此,深入了解微生物参与的周围神经-免疫调控机制,对创伤性脑损伤及其并发症的防御和治疗具有重要意义。此次综述全面概述了创伤性脑损伤后肠道微生态环境的变化,重点从脑-肠-微生物轴自上而下和自下而上的角度总结了创伤性脑损伤诱导的周围神经、免疫、微生物之间的复杂生物学过程,涉及自主神经功能障碍、神经内分泌紊乱、外周免疫抑制、肠屏障通透性增加、响应微生物的传入神经功能缺陷及中枢微生物潜在效应核团等机制的探索。此外,为更好的理解外周途径如何影响创伤性脑损伤患者的预后,文章还回顾了继发性生物损伤机制和损伤后的动态病理反应。文章提出与创伤性脑损伤恢复和未来中枢神经系统损伤类疾病风险相关的概念模型,揭示脑-肠-微生物轴双向影响的新见解。
https://orcid.org/0000-0003-2176-6657 (Yanjun Zhang); https://orcid.org/0000-0003-4308-3654 (Pengwei Zhuang)