NRR: 中国暨南大学陈功团队和中山大学孙逸仙纪念医院唐亚梅团队报道大脑原位神经再生技术治疗放射性脑损伤
#br#
撰文:闫旭东,钟科,陈功,唐亚梅,徐永腾
#br#
放射性脑损伤是头颈部肿瘤放疗后严重的并发症,临床表现为头痛、认知障碍或癫痫等症状,病理改变为脑组织水肿、坏死等,这严重影响患者的生活质量。目前放射性脑损伤的发病机制不明确,治疗手段有限,使得放射性脑损伤的临床防治成为领域内的重要挑战之一。中国中山大学孙逸仙纪念医院唐亚梅教授团队在放射性脑损伤领域深耕多年,在该疾病动物模型的构建、发病的机制以及治疗的策略等多方面均取得了诸多突破性进展 [1-8]。来自中国暨南大学的陈功教授团队使用原位神经再生技术将转录因子原位表达于受损脑区,在阿尔茨海默病、亨廷顿舞蹈症、颞叶癫痫和缺血性脑卒中等多种疾病的啮齿类或非人灵长类动物模型中进行了大量研究,取得了一系列研究成果,并刊登于国际权威期刊,为放射性脑损伤疾病提供了新的治疗策略 [9-16]。
最近,陈功和唐亚梅教授等在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“NeuroD1-based in situ neural regeneration for the treatment of radiation-induced brain
injury”的研究。该研究将神经细胞分化因子1(NeuroD1,ND1)原位注射于放射性脑损伤小鼠的脑部放射损伤区域,通过核磁共振和免疫荧光染色等手段发现ND1将损伤区域应激性星形胶质细胞转分化为神经元,且保护本底神经元,增加神经元密度,并减轻病灶区炎症反应,减少血管增生和外周 CD8+ T 细胞浸润,因此显著减小了损伤灶体积。本研究还通过高通量测序探索了其潜在机制。
核磁共振结果显示,放射性脑损伤小鼠在造模后10周时观察到明显的损伤灶,且使用ND1治疗后的小鼠损伤灶体积明显小于对照病毒组小鼠,证明ND1在表达后发挥治疗作用(图1A和B)。ND1的治疗不仅减小损伤灶的体积,还减少神经元的丢失(图1C和D)。在造模后10周时,ND1治疗组小鼠激活的星形胶质细胞约有36%转分化为神经元,体现为这些细胞既表达tdTomato信号,又表达NeuN信号;而对照组小鼠tdTomato阳性的细胞未发现与NeuN信号共标(图1E-G)。此外,ND1治疗组小鼠非tdTomato阳性的神经元密度高于对照病毒组小鼠,说明ND1的过表达还发挥对本底神经元的保护作用(图1 H)。
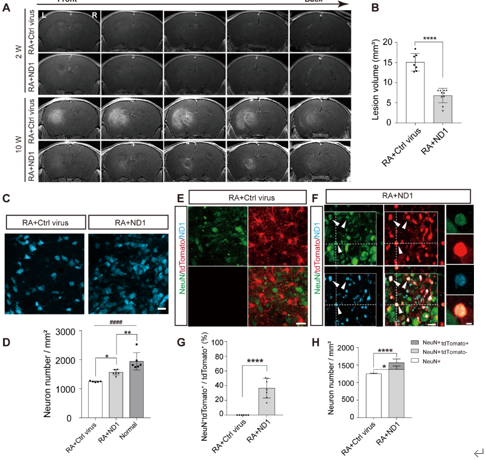
图1 原位过表达ND1减少放射性脑损伤小鼠放射损伤灶体积并通过诱导转分化发生增加神经元密度
ND1发挥如上治疗作用的原因可能是其表达改善了损伤灶周围的微环境。ND1治疗后小鼠损伤灶炎症反应低于对照病毒组小鼠:ND1治疗组小鼠Iba1密度较低且小胶质细胞具有更多分支,而对照病毒组小鼠小胶质细胞密度较高且突起回缩,呈现更圆的阿米巴样形态(图2A-C)。此外,ND1治疗组小鼠损伤灶CD8+ T细胞的密度也明显低于对照病毒组小鼠(图2D和E)。放射性脑损伤患者会出现明显的血管增殖现象,而增殖后的血管未形成完善的血脑屏障,导致脑水肿的发生和外周CD8阳性T细胞的浸润,而后者的浸润又会进一步加剧脑损伤的加重 [4, 17-20]。研究发现,ND1治疗组小鼠的血管密度和长度低于对照病毒组小鼠(图2F-H),说明ND1的过表达抑制了放射性脑损伤小鼠的血管增生。目前临床患者使用的放射性脑损伤治疗药物贝伐单抗也正是基于此治疗机制 [17]。而高通量测序的结果也证明了以上结论。该测序结果发现,ND1治疗上调了放射性脑损伤小鼠神经发生相关基因的表达,下调了血管增殖和免疫应答相关基因的表达,因此而实现上述治疗效果。
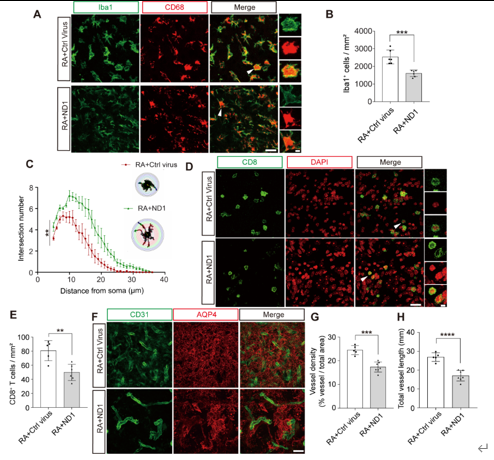
图2 ND1过表达改善损伤灶周围微环境
#br#
综上所述,陈功和唐亚梅教授等发现在放射性脑损伤小鼠中原位过表达转录因子ND1,以上调神经发生相关基因、下调血管增殖和免疫应答相关基因的方式,将损伤灶区域激活的星形胶质细胞转分化为神经元,并抑制血管增殖、降低炎症反应,从而保护本底神经元,减小损伤灶的体积,发挥治疗作用。
最近,美国食品和药物管理局加速通过了一种基于腺相关病毒为载体的基因疗法,其旨在通过单次原位颅内注射治疗芳香族 L-氨基酸脱羧酶缺乏症患者。其临床实验结果显示,早在治疗后3个月内,就已观察到患者运动功能的显著改善,并且能持续数年 [21]。基于ND1的原位神经再生技术已在放射性脑损伤模型小鼠中取得了较好的治疗效果,其同为原位注射的基因治疗手段,在放射性脑损伤的临床治疗方面具有较大潜力和广阔前景。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-01067
参考文献
#br#
[1] Cheng J, Jiang J, He B, et al. A phase 2 study of thalidomide for the treatment of radiation-induced blood-brain barrier injury. Sci Transl Med. 2023;15(684):eabm6543.
[2] Shi Z, Yu P, Lin WJ, et al. Microglia drive transient insult-induced brain injury by chemotactic recruitment of CD8(+) T lymphocytes. Neuron. 2023;111(5):696-710.e699.
[3] Xu Y, Rong X, Hu W, et al. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018;101(5):1087-1095.
[4] He B, Wang X, He Y, et al. Gamma ray-induced glial activation and neuronal loss occur before the delayed onset of brain necrosis. FASEB J. 2020;34(10):13361-13375.
[5] Jiang J, Li Y, Shen Q, et al. Effect of pregabalin on radiotherapy-related neuropathic pain in patients with head and neck cancer: a randomized controlled trial. J Clin Oncol. 2019;37(2):135-143.
[6] Zhang Z, Jiang J, He Y, et al. Pregabalin mitigates microglial activation and neuronal injury by inhibiting HMGB1 signaling pathway in radiation-induced brain injury. J Neuroinflammation. 2022;19(1):231.
[7] Ma X, Zuo Y, Hu X, et al. Terminally differentiated cytotoxic CD4(+) T cells were clonally expanded in the brain lesion of radiation-induced brain injury. CNS Neurosci Ther. 2024;30(3):e14682.
[8] Zhao X, Cheng J, Gui S, et al. Amifostine-loaded nanocarrier traverses the blood-brain barrier and prevents radiation-induced brain injury. ACS Appl Mater Interfaces. 2023;15(12):15203-15219.
[9] Guo Z, Zhang L, Wu Z, et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell. 2014;14(2):188-202.
[10] Chen YC, Ma NX, Pei ZF, et al. A NeuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Mol Ther. 2020;28(1):217-234.
[11] Ge LJ, Yang FH, Li W, et al. In vivo neuroregeneration to treat ischemic stroke through neurod1 aav-based gene therapy in adult non-human primates. Front Cell Dev Biol. 2020;8:590008.
[12] Wu Z, Parry M, Hou XY, et al. Gene therapy conversion of striatal astrocytes into GABAergic neurons in mouse models of Huntington's disease. Nat Commun. 2020;11(1):1105.
[13] Guo Y, Chen J, Ji W, et al. High-titer AAV disrupts cerebrovascular integrity and induces lymphocyte infiltration in adult mouse brain. Mol Ther Methods Clin Dev. 2023;31:101102.
[14] Ma NX, Puls B, Chen G. Transcriptomic analyses of NeuroD1-mediated astrocyte-to-neuron conversion. Dev Neurobiol. 2022;82(5):375-391.
[15] Xiang Z, He S, Chen R, et al. Two-photon live imaging of direct glia-to-neuron conversion in the mouse cortex. Neural Regen Res. 2024;19(8):1781-1788.
[16] He Q, Wang Z, Wang Y, et al. Characteristic changes in astrocyte properties during astrocyte-to-neuron conversion induced by NeuroD1/Ascl1/Dlx2. Neural Regen Res. 2025;20(6):1801-1815.
[17] Cai J, Zheng J, Shen J, et al. A radiomics model for predicting the response to bevacizumab in brain necrosis after radiotherapy. Clin Cancer Res. 2020;26(20):5438-5447.
[18] Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003;9(4):180-188.
[19] Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci. 2013;20(4):485-502.
[20] Wong CS, Van Der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4(5):273-284.
[21] Tai CH, Lee NC, Chien YH, et al. Long-term efficacy and safety of eladocagene exuparvovec in patients with AADC deficiency. Mol Ther. 2022;30(2):509-518.

