NRR:上海交通大学殷善开、时海波团队发现抑制NLRP3炎性小体可减轻氨基糖苷类药物所致耳蜗螺旋神经元的退行性变
撰文:方佳,李壮壮,王鹏军,邢雅智
耳蜗螺旋神经元退行性变是导致感音神经性耳聋的主要原因之一[1, 2]。目前治疗重度及极重度听力损失主要依赖于人工耳蜗植入,而螺旋神经元的存活和良好功能状态对人工耳蜗植入后的效果尤为重要。目前,螺旋神经元退行性变的机制仍然不清楚。氨基糖苷类药物可造成听功能的不可逆损伤,除毛细胞作为直接靶细胞发生凋亡外,螺旋神经元亦在经受氨基糖苷类药物处理后经历了不同的损伤/修复阶段,且损伤修复过程决定了残留螺旋神经元的功能状态。急性损伤期,细胞死亡反应主要为凋亡,受程序控制的细胞主动死亡过程,不产生炎性反应。而非炎性细胞凋亡不能完全解释螺旋神经元持续超过数月的严重损伤过程。细胞焦亡涉及损伤相关分子模式(DAMP)和病原相关分子模式分子对模式识别受体(PRR)的激活,以及炎性小体的组装、炎性半胱氨酸蛋白酶和Gasdermin D(GSDMD)的激活,以及白细胞介素1β和白细胞介素18的释放[3-6]。在中枢神经系统中,炎性小体在神经细胞中的激活累积与细胞焦亡诱导的神经元细胞死亡相关[7, 8]。然而在周围神经系统,特别是在听觉神经变性中,其作用未明确。因此,探索细胞焦亡及神经炎症通路在螺旋神经元退行性变中的作用具有重要意义。
来自中国上海交通大学医学院附属第六人民医院殷善开时海波团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“ Inhibition of the NLRP3 inflammasome attenuates spiral ganglion neuron degeneration in aminoglycoside-induced hearing loss” 的研究。该研究发现,NLRP3炎性小体的激活导致小鼠耳蜗螺旋神经元变性进行性加重。细胞焦亡在氨基糖苷类药物所致螺旋神经元退行性变中起重要作用。通过小分子化合物Mcc950或基因敲除抑制NLRP3均可显著减轻氨基糖苷类药物所致小鼠螺旋神经元退行性变,并改善其听功能。这是首次报道炎性小体活化组装后细胞焦亡通路激活是应用耳毒性药物后螺旋神经元退行性变加速发生的主要原因。研究结果为未来开发针对NLRP3的潜在治疗螺旋神经元退行性变提供了证据。
该研究首先观察了经典细胞焦亡通路在卡那霉素所致螺旋神经元退行性变中激活情况,Nlrp3,ASC,caspase-1和GSDMD的基因及蛋白水平均显著升高(图1)。卡那霉素所致螺旋神经元退行性变中,白细胞介素1β和白细胞介素18促进了神经炎症的发生(图2)。研究进一步发现卡那霉素所致螺旋神经元退行性变中伴随着巨噬细胞的活化和浸润(图3)。通过体外使用NLRP3炎性小体的特异性抑制剂Mcc950,抑制Nlrp3,ASC,caspase-1和GSDMD表达,减少白细胞介素1β和白细胞介素18分泌,减轻卡那霉素所致螺旋神经元损伤(图4)。为验证NLRP3缺失对螺旋神经元存活的影响,研究者对Nlrp3敲低小鼠进行了卡那霉素处理,比较了治疗前和治疗后15d的听力情况。在药物损伤后第15天,尽管2组小鼠的听力阈值均升高,在8和16 kHz频率处Nlrp3敲低小鼠的阈值低于野生型,螺旋神经元损伤程度亦较野生型减轻(图5)。这些结果表明,细胞焦亡在氨基糖苷药物所致螺旋神经元退行性变中起着重要作用,抑制NLRP3炎性小体可显著减轻螺旋神经元损伤。
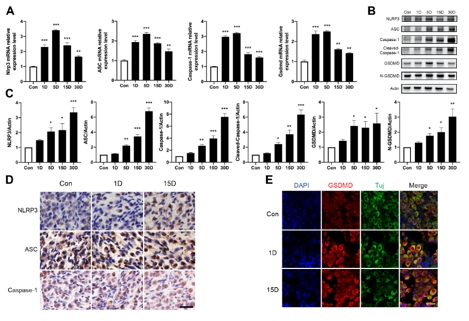
图1卡那霉素损伤后,螺旋神经元区域炎性小体及经典细胞焦亡通路显著激活(图源:Fang et al., Neural Regen Res, 2025)
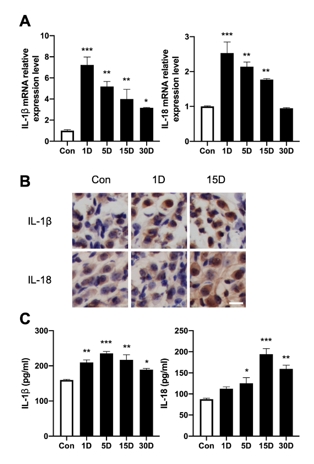
图2螺旋神经元损伤后神经炎症因子白细胞介素1β和白细胞介素18分泌增加(图源:Fang et al., Neural Regen Res, 2025)
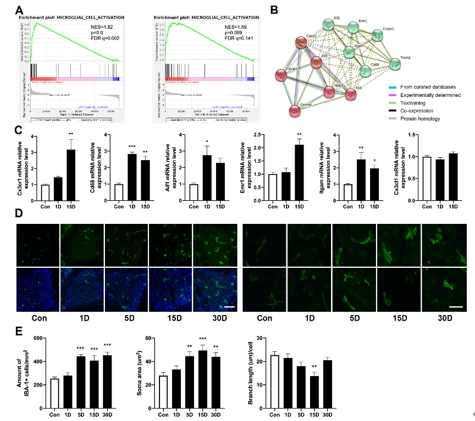
图3螺旋神经元损伤后伴随着组织内巨噬细胞的活化和浸润(图源:Fang et al., Neural Regen Res, 2025)
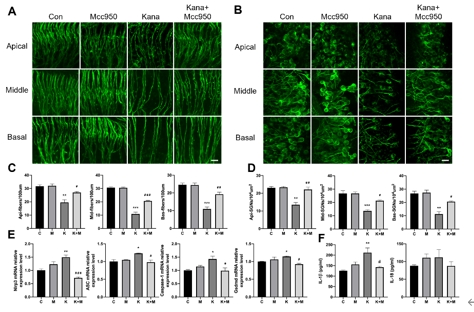
图4 Mcc950抑制NLRP3炎性小体形成,减轻卡那霉素造成的螺旋神经元损伤(图源:Fang et al., Neural Regen Res, 2025)
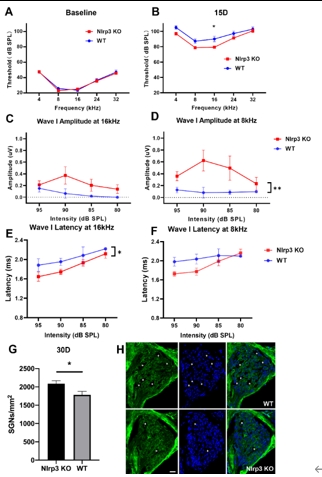
图5 Nlrp3基因敲除可部分拮抗卡那霉素所致螺旋神经元损伤(图源:Fang et al., Neural Regen Res, 2025)
总之,NLRP3炎性小体在氨基糖苷药物所致螺旋神经元退行性变中起着重要作用。作者的研究表明,经典细胞焦亡通路中的分子在卡那霉素所致螺旋神经元退行性变中显著升高,并持续较长时间,通过药物或基因干预手段抑制NLRP3炎性小体形成可显著减轻螺旋神经元损伤。此项研究将为拮抗螺旋神经元损伤提供潜在治疗方向。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01879
参考文献
[1] Wang M, Xu L, Han Y, et al. Regulation of spiral ganglion neuron regeneration as a therapeutic strategy in sensorineural hearing loss. Front Mol Neurosci. 2021;14:829564.
[2] Zhang L, Chen S, Sun Y. Mechanism and prevention of spiral ganglion neuron degeneration in the cochlea. Front Cell Neurosci. 2021;15:814891.
[3] Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113-114.
[4] Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812-1825.
[5] Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821-832.
[6] Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143-157.
[7] De Dios C, Abadin X, Roca-Agujetas V, et al. Inflammasome activation under high cholesterol load triggers a protective microglial phenotype while promoting neuronal pyroptosis. Transl Neurodegener. 2023;12(1):10.
[8] Von Herrmann KM, Salas LA, Martinez EM, et al. NLRP3 expression in mesencephalic neurons and characterization of a rare NLRP3 polymorphism associated with decreased risk of Parkinson's disease. NPJ Parkinsons Dis. 2018;4:24.




