NRR:宁波大学医学部李丽萍团队揭示跑步运动联合声光嗅觉刺激作为治疗老年痴呆的非药物干预策略
撰文:肖彪,李丽萍
阿尔茨海默病是一种常见的神经退行性疾病,以进行性记忆丧失和认知障碍为特征。迄今为止,全世界尚无阿尔茨海默病的有效治疗药物及治疗方法 [1],因此探索阿尔茨海默病的潜在非药物干预策略至关重要。单一因素干预形式(如跑步运动、声光刺激、嗅觉刺激等)被证实可一定程度改善老年阿尔茨海默病小鼠的脑部淀粉样蛋白β(amyloid-beta,Aβ)负荷和认知水平 [2, 3],然而单一因素干预的脑区范围、脑部深度、治疗效果的维持时间有限 [4]。因此,探索多因素联合干预—跑步运动联合声光嗅觉刺激可能引起更广泛的全身系统性影响,从而高效、持久的治疗阿尔茨海默病。
来自中国宁波大学医学部李丽萍团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Treadmill exercise in combination with acousto-optic and olfactory
stimulation improves cognitive function in APP/PS1 mice through the
brain-derived neurotrophic factor- and Cygb-associated signaling pathways”的研究揭示了跑步运动联合声光和嗅觉刺激的多因素干预在成年海马神经发生、Aβ负荷、认知水平、终止治疗后维持时间等中的作用,阐述了多因素干预在防治阿尔茨海默病的新机制,为早期阿尔茨海默病的非药物干预策略提供了新思路。
海马神经干细胞的增殖和分化在大脑功能中发挥着重要作用,参与记忆、抑郁等行为 [5]。Aβ斑块在阿尔茨海默病的发生发展中被认为是主要角色[6]。患者在出现行为症状之前,大脑就已经出现阿尔茨海默病相关的病理变化,因此探清不同阶段阿尔茨海默病病理发展过程,为阿尔茨海默病的早期干预寻找合适时机。李丽萍等首先探究了不同阶段APP/PS1小鼠海马神经发生和Aβ负荷的变化,发现APP/PS1小鼠海马中的BrdU+细胞、DCX+细胞、BrdU+DCX+和BrdU+GFAP+细胞的数量随着年龄增加和病情进展而减少(图1),并伴随着的斑块沉积显著增加。APP/PS1小鼠海马在4月龄时出现小而零星的Aβ斑块沉积,此时神经细胞处于旺盛的增殖阶段,而6月龄后海马出现大而致密的斑块沉积,神经细胞增殖能力开始出现了衰退。结果表明4月龄的APP/PS1小鼠海马出现少量斑块沉积但未出现神经发生的损伤,而6月龄后随着Aβ斑块富集增多神经发生开始衰竭。作者选择APP/PS1小鼠海马斑块刚出现(4月龄)和神经发生开始衰竭(6月龄)的两个重要时间点分别进行多因素干预。发现持续4周的多因素干预均能增加了4和6月龄APP/PS1小鼠海马中BrdU+细胞、DCX+细胞、BrdU+DCX+细胞、BrdU+GFAP+细胞和BrdU+NeuN+细胞的数量(图2),并减少Aβ的负荷。但是4月龄组干预的治疗效果显著优于6月龄组,提示多因素干预可促进成年APP/PS1小鼠海马神经再生和降低Aβ水平,且早期干预效果更为显著。

图1 APP/PS1小鼠海马神经细胞增殖和分化随年龄增加和阿尔茨海默病进展而下降(图源:Xiao et al., Neural Regen Res, 2025)
 #br#
#br#
图2 多因素干预可促进APP/PS1小鼠海马的成年神经发生(图源:Xiao et al., Neural Regen Res, 2025)
由于神经再生和Aβ负荷与行为表现相关,李丽萍等通过行为学实验评估了多因素干预对物体辨认能力、空间记忆能力和抑郁样行为表现的影响,发现持续4周的多因素干预可以提高APP/PS1小鼠对物体的辨认能力、降低空间记忆的障碍和抑郁样行为的风险(图3)。
 #br#
#br#
#br#
图3多因素干预提高APP/PS1小鼠对物体辨认能力和降低抑郁样行为的风险(图源:Xiao et
al., Neural Regen Res, 2025)
李丽萍等进一步评估多因素干预的疗效持续时间。在停止治疗2周后,小鼠海马DCX+的细胞数量仍显著增加,表明多因素干预在提升4月龄APP/PS1小鼠海马DCX负荷中疗效可至少维持2周,提示多因素干预在治疗结束后可至少维持2周。

图4停止治疗2周后,多因素干预仍维持APP/PS1小鼠海马神经发生(图源:Xiao et
al., Neural Regen Res, 2025)
为进一步探明多因素干预对阿尔茨海默病小鼠海马神经再生影响的分子机制。研究人员发现多因素干预通过脑源性神经营养因子和细胞红蛋白介导的信号通路产生促进神经发生、抑制细胞凋亡、抗氧化损伤的治疗作用。通过血清代谢组测序分析,发现干预治疗后APP/PS1小鼠血清中出现55个代谢产物受到干预的调控,这些代谢物主要参与细胞死亡、抗氧化损伤等生物途径(图5)。
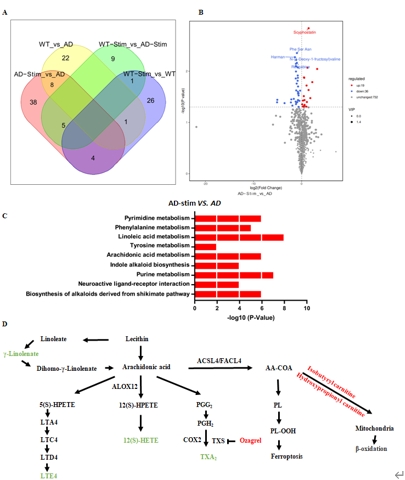
图5代谢物测序揭示了APP/PS1小鼠血清样品中受多因素刺激调控的差异表达代谢物,且主要参与细胞凋亡和抗氧化等生物过程(图源:Xiao et
al., Neural Regen Res, 2025)
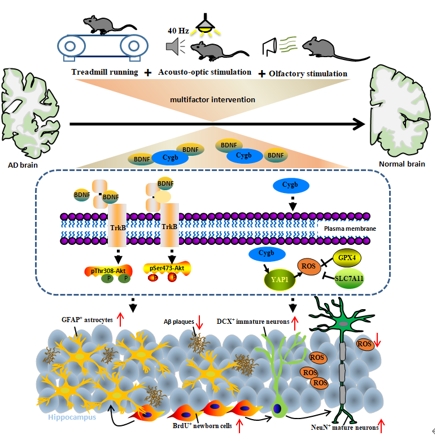
综上所述,多因素干预——跑步运动联合声光和嗅觉刺激可以促进成年APP/PS1小鼠海马神经再生,降低Aβ的负荷,降低认知障碍和精神症状的风险。治疗结束后,干预的疗效至少维持2周。早期进行多因素干预更有利于防止阿尔茨海默病后期出现的神经再生衰退和行为障碍。本研究证明跑步运动联合声光和嗅觉刺激的多因素干预对阿尔茨海默病的治疗效果,揭示了多因素干预治疗阿尔茨海默病的作用机制(图6),这一发现丰富了非药物干预对阿尔茨海默病防治的策略。但是研究仅探索了多因素干预在阿尔茨海默病动物模型中的治疗效果,没有对临床的应用效果进一步验证。由于阿尔茨海默病患者病理的复杂性,仍需进一步的研究才能完全理解其临床应用价值。40Hz声光刺激被证明通过恢复阿尔茨海默病小鼠脑部伽马振荡而达到治疗效果,但遗憾的是,研究没有进一步通过电生理手段检测多因素干预是否引起脑电活动变化(尤其是伽马振荡)。此外,多因素刺激调控血清代谢物和组织目标蛋白质之间的内在联系在预治阿尔茨海默病中的作用也亟待进一步阐明。
 #br#
#br#
图6跑步运动联合声光和嗅觉刺激的多因素干预治疗阿尔茨海默病的作用机制(图源:Xiao et
al., Neural Regen Res, 2025)
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01681
参考文献
[1] Liu JY, Guo HY, Quan ZS, et al.
Research progress of natural products and their derivatives against Alzheimer's
disease. J Enzyme Inhib Med Chem. 2023;38(1):2171026.
[2] Park SS, Park HS, Kim CJ, et al.
Physical exercise during exposure to 40-Hz light flicker improves cognitive
functions in the 3xTg mouse model of Alzheimer's disease. Alzheimers Res Ther.
2020;12(1):62.
[3] Sagud M, Tudor L, Pivac N.
Personalized treatment interventions: nonpharmacological and natural treatment
strategies in Alzheimer's disease. Expert Rev Neurother. 2021;21(5):571-589.
[4] Martorell AJ, Paulson AL, Suk HJ, et
al. Multi-sensory gamma stimulation ameliorates Alzheimer's-associated
pathology and improves cognition. Cell. 2019;177:256-271.e22(2).
[5] Anacker C, Luna VM, Stevens GS, et al.
Hippocampal neurogenesis confers stress resilience by inhibiting the ventral
dentate gyrus. Nature. 2018;559(7712):98-102.
[6] Huang YR, Xie XX, Yang J, et al.
ArhGAP11A mediates amyloid-β generation and neuropathology in an Alzheimer's
disease-like mouse model. Cell Rep. 2023;42(6):112624.
#br#
#br#

 #br#
#br#
 #br#
#br#


 #br#
#br#