NRR:中国中山大学王艺东研究团队揭示鞘内移植人脐带间充质干细胞治疗缺血性脑卒中的可行性
缺血性脑卒中后狭窄的再灌注治疗时间窗导致只有很小比例的患者从治疗中获益。研究表明干细胞通过促进神经突触重塑、神经新生、血管新生等机制发挥诱导神经修复的潜力[1],因而已成为脑梗死治疗的新的希望。与其他类型或其他来源干细胞相比,人脐带间充质干细胞因其几乎无成瘤可能、细胞来源较为丰富、分离纯化操作相对简便、不存在伦理问题、呈免疫豁免状态等特点而具有临床使用的优势[2]。既往临床前研究已对通过不同移植途径移植间充质干细胞治疗缺血性脑卒中进行了一些探索[3-7],然而在大鼠永久性大脑中动脉阻塞模型通过小脑延髓池移植人脐带间充质干细胞的安全性和有效性尚无研究。
中国中山大学孙逸仙纪念医院王艺东教授团队在《中国神经再生研究(英文)》(Neural Regeneration Research)发表的研究发现,永久性大脑中动脉阻塞大鼠通过小脑延髓池鞘内注射人脐带间充质干细胞不仅安全而且有效,涉及的机制可能包括减轻局部炎症反应、促进神经新生和血管新生以及增强神经营养因子的产生。黄泽嘉和姜交华为论文共同第一作者,王艺东和陈海佳为共同通讯作者。
缺血性脑卒中的病理生理学过程涉及局部脑组织的缺血、神经元损失、氧化应激反应、炎症免疫反应等,共同导致神经功能损伤[8]。为模拟临床常见的缺血性脑卒中情形,该研究建立了永久性大脑中动脉阻塞大鼠模型,在建模后72小时通过小脑延髓池注射生理盐水或不同剂量人脐带间充质干细胞(5×105和1×106)。结果显示,与注射生理盐水的对照组相比,鞘内移植人脐带间充质干细胞并未增加大鼠的死亡率,也未引起一般状况、行为活动或组织病理上的不良改变;人脐带间充质干细胞移植显著减少了梗死体积并促进了神经功能的恢复,且在不同剂量人脐带间充质干细胞间未观察到显著性差异(图1)。该研究进一步发现,人脐带间充质干细胞移植增加了梗死周围区域的神经元数量,并减少了小胶质细胞和星形胶质细胞的活化(图2)。该结果与先前研究结果一致[9],证实了MSCs具有免疫调节功能,能够减轻炎症反应,保护神经元免受大脑中动脉阻塞引起的损伤。此外,与既往研究 [10,11] 有类似的发现,人脐带间充质干细胞促进了梗死周围区CD31+微血管、CD31+/Ki67+细胞的数量增加,以及室管膜下区和齿状回颗粒细胞层下区DCX阳性细胞的表达,表明其对血管生成和神经新生具有促进作用(图3)。脑源性神经营养因子的表达在人脐带间充质干细胞移植后显著增加,且脑脊液中脑源性神经营养因子水平在移植后14天和28天均有显著提高,该结果强调了脑源性神经营养因子在神经网络形成中的潜在重要作用。
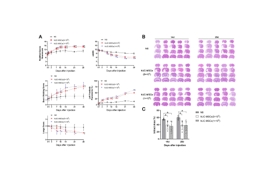
图1 鞘内移植人脐带间充质干细胞促进了神经功能恢复和显著减少梗死体积
(图源:Huang Z., et al. Neural Regen Res, 2026)
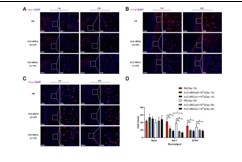
图2 鞘内移植人脐带间充质干细胞增加了梗死周围区神经元的数量,同时减少了小胶质细胞和星形胶质细胞的数量
(图源:Huang Z., et al. Neural Regen Res, 2026)
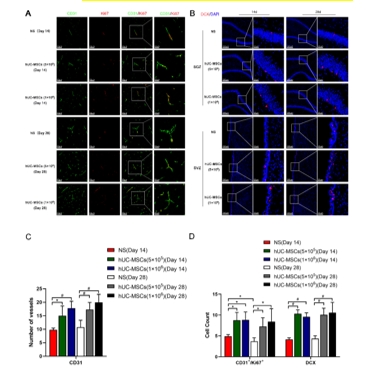
图3 鞘内移植人脐带间充质干细胞促进了血管生成和神经新生
(图源:Huang Z., et al. Neural Regen Res, 2026)
#br#
综上所述,永久性大脑中动脉阻塞大鼠鞘内移植人脐带间充质干细胞安全且有效,可显著减少脑梗死体积,促进神经功能恢复。此项研究结果为人脐带间充质干细胞鞘内移植治疗缺血性脑卒中提供了更多的临床前证据。
当然该研究也存在一些局限性。首先,该研究只观察了两种剂量的人脐带间充质干细胞在治疗效果上没有显著性差异,有必要进一步探索更低剂量人脐带间充质干细胞的疗效,以确定是否存在更小的有效剂量,从而在保证有效性的前提下减少所需的干细胞剂量。其次,缺血性脑卒中是病理生理机制极其复杂的疾病,涉及多种细胞类型、信号通路和分子介质。该研究没有深入探讨人脐带间充质干细胞对相关信号通路的影响。为了更全面地理解人脐带间充质干细胞如何发挥作用,未来的研究需要从分子层面进行更细致的研究。
#br#
参考文献:
[1] Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466-473.
[2] Rahman MM, Islam MR, Islam MT, et al. Stem cell transplantation therapy and neurological disorders: current status and future perspectives. Biology (Basel). 2022;11:147.
[3] Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38.
[4] Noh JE, Oh SH, Park IH, et al. Intracerebral transplants of GMP-grade human umbilical cord-derived mesenchymal stromal cells effectively treat subacute-phase ischemic stroke in a rodent model. Front Cell Neurosci. 2020;14:546659.
[5] Guo Y, Peng Y, Zeng H, et al. Progress in mesenchymal stem cell therapy for ischemic stroke. Stem Cells Int. 2021;2021:9923566.
[6] Namestnikova DD, Gubskiy IL, Revkova VA, et al. Intra-arterial stem cell transplantation in experimental stroke in rats: real-time MR visualization of transplanted cells starting with their first pass through the brain with regard to the therapeutic action. Front Neurosci. 2021;15:641970.
[7] Yarygin KN, Namestnikova DD, Sukhinich KK, et al. Cell therapy of stroke: do the intra-arterially transplanted mesenchymal stem cells cross the blood-brain barrier? Cells. 2021;10):2997.
[8] Zhao Y, Zhang X, Chen X, et al. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int J Mol Med. 2022;49:15.
[9] Kong T, Park JM, Jang JH, et al. Immunomodulatory effect of CD200-positive human placenta-derived stem cells in the early phase of stroke. Exp Mol Med. 2018;50:e425.
[10] Lalu MM, Montroy J, Dowlatshahi D, et al. From the lab to patients: a systematic review and meta-analysis of mesenchymal stem cell therapy for stroke. Transl Stroke Res. 2020;11:345-364.
[11] Pirzad Jahromi G, Shabanzadeh Pirsaraei A, Sadr SS, et al. Multipotent bone marrow stromal cell therapy promotes endogenous cell proliferation following ischemic stroke. Clin Exp Pharmacol Physiol. 2015;42:1158-1167.


