中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (11): 2545-2552.doi: 10.4103/1673-5374.371374
• 原著:神经损伤修复保护与再生 • 上一篇
神经病理性疼痛后m6A甲基化的表观遗传学及转录组学分析
Epigenetic combined with transcriptomic analysis of the m6A methylome after spared nerve injury-induced neuropathic pain in mice
Fanning Zeng1, 2, Jun Cao1, 3, Zexuan Hong1, 2, Yitian Lu1, Zaisheng Qin1, *, Tao Tao2, *
- 1Department of Anesthesiology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong Province, China; 2Department of Anesthesiology, Central People’s Hospital of Zhanjiang, Zhanjiang, Guangdong Province, China; 3Department of Anesthesiology, Shenzhen Maternity & Child Healthcare Hospital, Southern Medical University, Shenzhen, Guangdong Province, China
摘要:
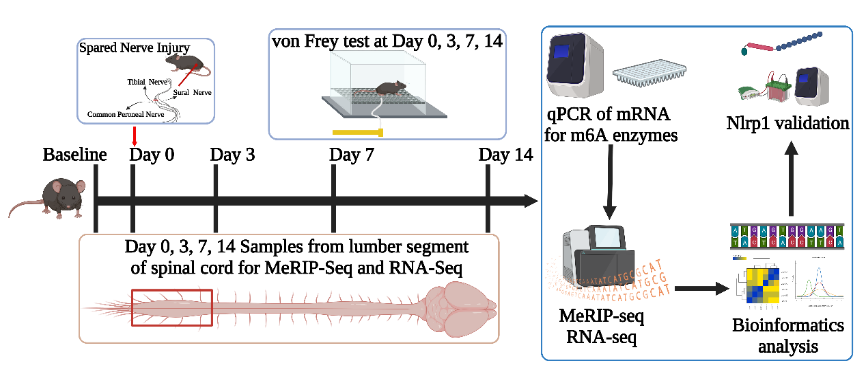
脊髓中的表观遗传学变化在周围神经损伤引起的神经病理性疼痛的发生和维持中起着重要的作用。N6甲基腺苷(m6A)修饰是最常见的RNA修饰之一,在多种疾病的基因调控中起着重要的作用。然而,神经病理性疼痛不同阶段脊髓中mRNA整体m6A修饰状态仍不清楚。此次实验以保留完整的腓肠神经而只损伤腓总神经的方法建立了神经病理性疼痛模型。RNA 免疫共沉淀结合高通量测序结果显示,周围神经损伤后脊髓中存在55种m6A甲基化且差异表达的基因。基因本体论(GO)和京都基因百科全书(KEGG)通路分析结果表明,m6A修饰可触发周围神经损伤后早期的炎症反应和凋亡。且随着时间的推移,m6A甲基化的差异基因可导致突触形态可塑性改变,这成为神经病理性疼痛发生和维持的转折点。神经病理性疼痛的持续存在是由富含m6A甲基化差异基因调节的脂质代谢过程引起的。其中,m6A甲基化修饰酶的甲基转移酶类和去甲基酶类在神经病理性疼痛中具有重要的调控作用,而该研究则发现阅读蛋白酶类修饰酶同样发挥了重要的作用。上述结果展示了神经病理性疼痛不同阶段脊髓mRNA的m6A甲基化的整体情况。
https://orcid.org/0000-0002-6348-719X (Tao Tao); https://orcid.org/0000-0001-7798-1423 (Zaisheng Qin)