中国神经再生研究(英文版) ›› 2025, Vol. 20 ›› Issue (11): 3302-3316.doi: 10.4103/NRR.NRR-D-23-01876
空间转录组学结合单核RNA测序揭示人类脊髓神经胶质细胞的异质性
Spatial transcriptomics combined with single-nucleus RNA sequencing reveals glial cell heterogeneity in the human spinal cord
Yali Chen1, 2, #, Yiyong Wei3, 4, #, Jin Liu1, 2, Tao Zhu1 , Cheng Zhou2, *, Donghang Zhang1, 2, *
- 1 Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China; 2 Laboratory of Anesthesia and Critical Care Medicine, National-Local Joint Engineering Research Center of Translational Medicine of Anesthesiology, West China Hospital, Sichuan University, Chengdu, Sichuan Province, China; 3 Department of Anesthesiology, Longgang District Maternity & Child Healthcare Hospital of Shenzhen City (Longgang Maternity and Child Institute of Shantou University Medical College), Shenzhen, Guangdong Province, China; 4 Department of Anesthesiology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou Province, China
摘要:
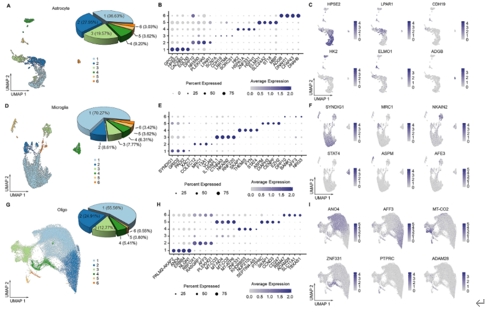
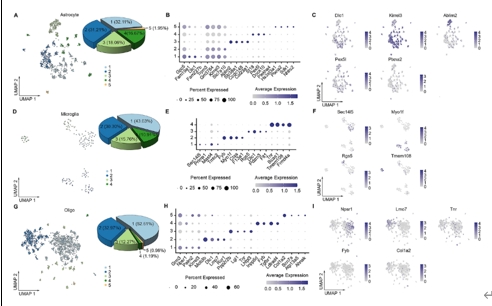
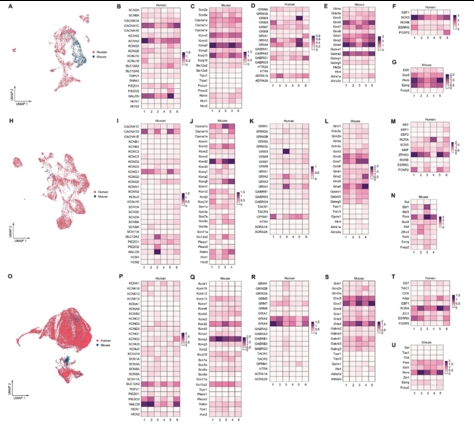
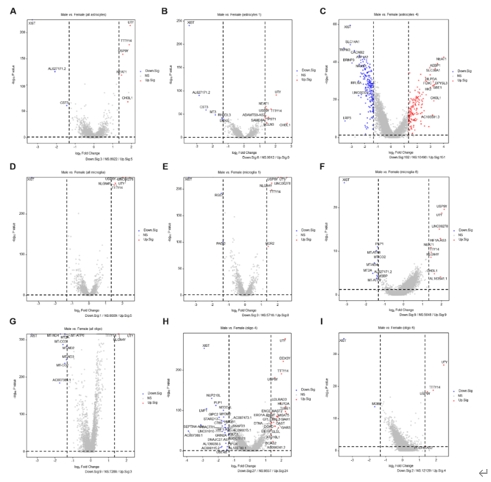
胶质细胞在调节生理功能以及感觉、感染、急性损伤和慢性神经退行性疾病等疾病的病理变化方面发挥着至关重要的作用。胶质细胞包括中枢神经系统中的星形胶质细胞、小胶质细胞和少突胶质细胞,以及周围神经系统中的卫星胶质细胞和许旺细胞。尽管通过单细胞RNA测序或单核RNA测序已对动物模型中的神经胶质亚型和功能异质性有所了解,但很少有研究关注人类脊髓中神经胶质细胞的转录组学特征。此次实验使用空间转录组学结合高通量单核RNA测序来绘制人类脊髓中星形胶质细胞、小胶质细胞和少突胶质细胞的细胞和分子异质性,同时为探索不同物种之间的差异,将这些人类与小鼠的神经胶质细胞保守和差异表达谱进行比较。在人类脊髓中星形胶质细胞、小胶质细胞和少突胶质细胞均被分为6个不同的转录组亚簇,而小鼠脊髓中星形胶质细胞、小胶质细胞和少突胶质细胞分别被分为5个、4个和5个不同的转录组亚簇。表明人类和小鼠所有神经胶质细胞都存在显著的异质性。此外,实验还检测了人类不同性别脊髓神经胶质细胞的基因表达差异。可见在所有的星形胶质细胞中,男性的NEAT1和CHI3L1水平高于女性,而CST3水平低于女性。对于所有的小胶质细胞,所有的差异基因都位于性别染色体上。除性别特异性基因外,女性脊髓中所有少突胶质细胞比男性含有更多的MT-ND4,MT2A,MT-ATP6,MT-CO3,MT-ND2,MT-ND3和MT-CO2。上述结果广泛表征了神经胶质细胞的异质性,并为探索慢性疼痛、肌萎缩侧索硬化症、多发性硬化等脊髓相关疾病的细胞基础提供了宝贵的资源。
https://orcid.org/0000-0002-2754-7204 (Donghang Zhang); https://orcid.org/0000-0002-5005-2285 (Cheng Zhou)