NRR:中国山西医科大学陆利和张承武团队利用转录组学与机器学习分析探索参与阿尔茨海默病颞中回血管功能障碍的新基因
撰文:王美玲,贺澳杰,康毓冰,王昭君,贺雅慧,林嘉隆,张承武,陆利
阿尔茨海默病是最常见的神经退行性疾病,也是老年痴呆症的主要原因,尽管已经进行了广泛的研究来了解导致阿尔茨海默病的大脑变化,但是其病因尚不清楚[1]。颞中回在阿尔茨海默病病理发展进程受到影响,是阿尔茨海默病患者的脑萎缩发生区域之一[2]。阿尔茨海默病病理与大脑微血管功能障碍和/或变性、神经血管解体、血脑屏障功能缺陷和/或血管因素有关[3]。最近的证据表明,血管功能障碍会导致神经元功能障碍和神经变性,进一步导致阿尔茨海默病患者认知功能衰退[3]。改善或预防阿尔茨海默病患者的血管功能障碍具有重要意义,但是目前缺乏阿尔茨海默病患者颞中回皮层血管功能障碍的全面分析。更深一步剖析阿尔茨海默病患者颞中回脑区与血管功能相关分子特征,将有助于深入探索阿尔茨海默病的病理机制,并推动其诊断和治疗的进一步发展。
来自中国山西医科大学陆利/张承武团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Novel genes involved in vascular dysfunction of the middle temporal gyrus in Alzheimer’s disease: transcriptomics combined with machine learning analysis”的研究,发现阿尔茨海默病患者颞中回内皮细胞和周细胞间通讯发生了显著地改变,且这与血管功能有关。此外,通过机器学习筛选出了阿尔茨海默病患者颞中回和血管功能有关的的4种新基因。这项研究深入揭示了阿尔茨海默病的发病机制,并为阿尔茨海默病治疗提供了新的靶点。
尽管阿尔茨海默病的病因尚未完全阐明,但血管功能障碍与阿尔茨海默病的发病机制有着强烈的相关性[4]。颞中回是大脑中在阿尔茨海默病中表现出显著损伤的区域之一[2],这提示在颞中回中涉及血管异常的分子将对阐明阿尔茨海默病的机制和发现新的干预靶点具有重大意义。陆利/张承武等首先对阿尔茨海默病患者以及健康对照者颞中回进行了单细胞转录测序功能富集分析,发现阿尔茨海默病颞中回血管功能发生了显著地改变(图1)。而后行细胞互作分析,发现细胞相互作用数量减少,其中内皮细胞和周细胞改变最为显著(图2),这2种细胞都和血管功能相关[5, 6]。与上述结果一致,还有其他团队也指出内皮细胞或周细胞内细胞间通讯受损会加速阿尔茨海默病的病理改变,包括脑血流量减少和血脑屏障通透性增加[7-9]。这些证据指向阿尔茨海默病患者颞中回脑区血管功能发生显著改变尤其是与VEGFA-VEGFR2信号改变直接相关。接下来通过AUCell分析鉴定出与该信号通路活性直接相关的内皮细胞与周细胞亚类,得出阿尔茨海默病颞中回发生显著改变的2类细胞亚类分别是Erb-B2受体酪氨酸激酶4(Erb-b2 receptor tyrosine kinase 2,ERBB4)高表达的内皮细胞(ERBB4high)和血管生成素样4(Angiopoietin-like 4,ANGPTL4)高表达的周细胞(ANGPTL4high)亚类(图3),上述数据为内皮细胞和周细胞亚类在阿尔茨海默病中的作用提供了新的证据。最后结合批量RNA测序数据以及2种机器学习算法筛选出4种特征基因,即生长抑素(Somatostatin, SST)、蛋白酪氨酸磷酸酶非受体3型(Protein tyrosine phosphatase non-receptor type 3, PTPN3)、谷氨酰胺酶(Glutaminase, GLS)和原肌凝蛋白3(Tropomyosin 3, TPM3),这些基因在阿尔茨海默病颞中回中下调(图4)。有趣的是,结合实验数据以及文献发现这4种特征基因(SST, PTPN3, GLS, TPM3)都可以靶向血管内皮生长因子(vascular endothelial growth factor, VEGF)通路[10-13]。一些研究也指出VEGF通路在阿尔茨海默病中发生改变,靶向该通路可以缓建阿尔茨海默病相关症状[14, 15]。综上提示,通过调控这些新靶点基因可能影响血管内皮生长因子通路,从而缓解或治疗阿尔茨海默病。
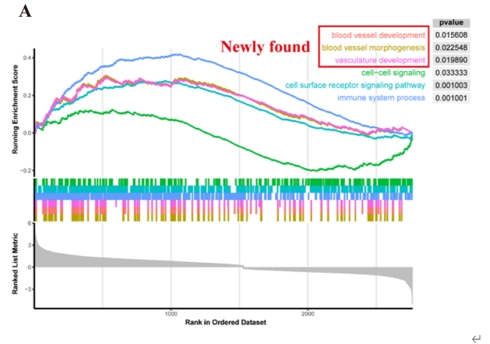
图1阿尔茨海默病患者颞中回脑区血管功能显著改变(图源:Wang et al., Neural Regen Res, 2025)
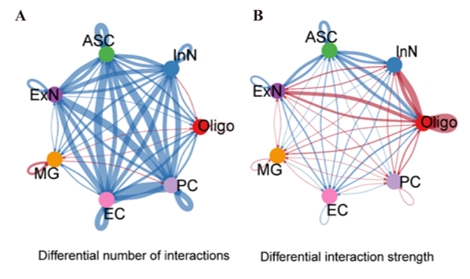
图2阿尔茨海默病患者颞中回脑区内皮细胞和周细胞相互作用数量减少(图源:Wang et al., Neural Regen Res, 2025)
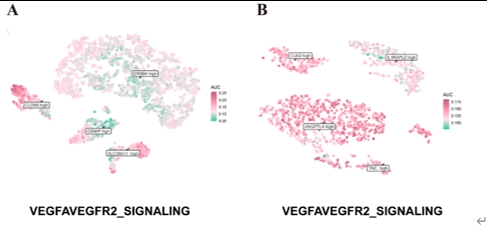
图3 ERBB4high内皮细胞和ANGPTL4high周细胞亚类中VEGFA-VEGFR2信号改变显著(图源:Wang et al., Neural Regen Res, 2025)
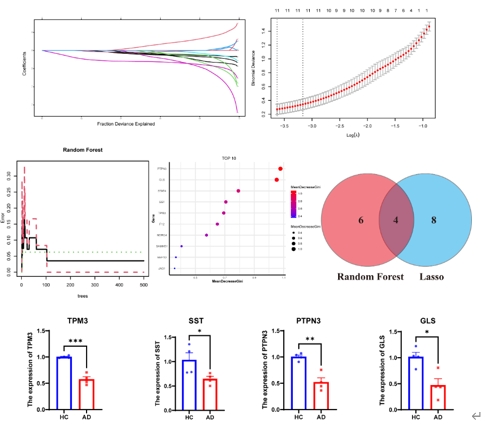
图4 阿尔茨海默病患者颞中回脑区四个与血管功能相关基因SST,PTPN3,GLS和TPM3显著下调(图源:Wang et al., Neural Regen Res, 2025)
陆利/张承武等利用单细胞数据集精心构建了阿尔茨海默病颞中回的全面转录组图谱。结果显示,阿尔茨海默病颞中回的血管功能受到了损害。此外还构建了机器学习模型来筛选参与调控血管功能的特征基因,并发现了4种新的相关基因。这不仅揭示了阿尔茨海默病发病的内在机制,也为阿尔茨海默病治疗学提供了新的线索。但是,此研究仍有一些局限性需要注意。一方面,scRNA-seq数据来源于有限的样本,而批量 RNA-seq 数据无法提供精确的细胞注释。另一方面,缺乏临床样本和这4种基因的蛋白质水平验证。通过综合分析筛选阿尔茨海默病大脑中颞中回的特征基因是探索性的,需要进行更详细的研究,以更好地了解阿尔茨海默病的内在机制。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-02004
参考文献
[1] De La Torre J. The Vascular Hypothesis of Alzheimer's disease: a key to preclinical prediction of dementia using neuroimaging. J Alzheimers Dis. 2018;63(1):35-52.
[2] Li K, Qu H, Ma M, et al. Correlation between brain structure atrophy and plasma amyloid-β and phosphorylated tau in patients with Alzheimer's disease and amnestic mild cognitive impairment explored by surface-based morphometry. Front Aging Neurosci. 2022;14:816043.
[3] Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. 2011;12(12):723-738.
[4] Cruz Hernández JC, Bracko O, Kersbergen CJ, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat Neurosci. 2019;22(3):413-420.
[5] Lei S, Li J, Yu J, et al. Porphyromonas gingivalis bacteremia increases the permeability of the blood-brain barrier via the Mfsd2a/Caveolin-1 mediated transcytosis pathway. Int J Oral Sci. 2023;15(1):3.
[6] Denes A, Hansen CE, Oezorhan U, et al. Endothelial cells and macrophages as allies in the healthy and diseased brain. Acta Neuropathol. 2024;147(1):38.
[7] Tureckova J, Kamenicka M, Kolenicova D, et al. Compromised astrocyte swelling/volume regulation in the hippocampus of the triple transgenic mouse model of Alzheimer's disease. Front Aging Neurosci. 2021;13:783120.
[8] Liu A, Fernandes BS, Citu C, et al. Unraveling the intercellular communication disruption and key pathways in Alzheimer’s disease: an integrative study of single-nucleus transcriptomes and genetic association. Alzheimers Res Ther. 2024;16(1):3.
[9] Choi YK. Role of carbon monoxide in neurovascular repair processing. Biomol Ther (Seoul). 2018;26(2):93-100.
[10] Bocci G, Culler MD, Fioravanti A, et al. In vitro antiangiogenic activity of selective somatostatin subtype-1 receptor agonists. Eur J Clin Invest. 2007;37(9):700-708.
[11] Mehta V, Fields L, Evans IM, et al. VEGF (vascular endothelial growth factor) induces NRP1 (neuropilin-1) cleavage via ADAMs (a disintegrin and metalloproteinase) 9 and 10 to generate novel carboxy-terminal NRP1 fragments that regulate angiogenic signaling. Arterioscler Thromb Vasc Biol. 2018;38(8):1845-1858.
[12] Cammalleri M, Bagnoli P, Bigiani A. Molecular and cellular mechanisms underlying somatostatin-based signaling in two model neural networks, the retina and the hippocampus. Int J Mol Sci. 2019;20(10):2506.
[13] Zhang S, Zhang R, Xu R, et al. MicroRNA-574-5p in gastric cancer cells promotes angiogenesis by targeting protein tyrosine phosphatase non-receptor type 3 (PTPN3). Gene. 2020;733:144383.
[14] Alvarez-Vergara MI, Rosales-Nieves AE, March-Diaz R, et al. Non-productive angiogenesis disassembles Aß plaque-associated blood vessels. Nat Commun. 2021;12(1):3098.
[15] Jeon SG, Lee HJ, Park H, et al. The VEGF inhibitor vatalanib regulates AD pathology in 5xFAD mice. Mol Brain. 2020;13(1):131.



