NRR:中国复旦大学杨雄里和钟咏梅研究团队揭示滴眼给予胰高血糖素样肽1可有效改善早期糖尿病视网膜神经节细胞功能
撰文:邵昱琦,钟咏梅
糖尿病性视网膜病变是糖尿病最常见和最特殊的并发症,也是导致成人视力损伤和失明的最主要病因[1]。视网膜神经节细胞丢失是糖尿病性视网膜病变发病的早期事件,与视觉功能障碍或失明相关[2]。由于糖尿病性视网膜病变的发病机制复杂,目前仍缺乏有效的治疗策略。已有研究表明,作为能够促进胰岛素分泌而有效控制血糖的“明星”—胰高血糖素样肽1及其受体激动剂,可改善糖尿病引起的视网膜神经退行性病变[3-5]。然而,对于这种保护作用的潜在神经机制知之甚少。
中国复旦大学脑科学研究院钟咏梅研究员、杨雄里院士和翁史钧研究员科研团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Topical administration of GLP-1 eyedrops improves retinal ganglion cell function by facilitating presynaptic GABA release in early experimental diabetes”的研究,发现滴眼局部给予胰高血糖素样肽1,能够通过促进无长突细胞至视网膜神经节细胞的γ-氨基丁酸释放,使视网膜神经节细胞相关的神经回路去兴奋(de-excitation),抑制兴奋性毒性过程,进而促进视网膜神经节细胞的存活及视觉功能的改善。这些结果提示,γ-氨基丁酸系统可能成为抑制早期糖尿病性视网膜病变的潜在治疗靶点,而滴眼给予胰高血糖素样肽1可以提供一种无创、有效的方法来治疗视网膜病变。
钟咏梅等首先使用全细胞膜片钳技术在正常大鼠视网膜切片标本上分离并记录视网膜神经节细胞的微小抑制性突触后电流(miniature inhibitory postsynaptic current)(图1A)。结果显示,胞外灌流胰高血糖素样肽1显著增加ON型视网膜神经节细胞(图1B-E)和OFF型视网膜神经节细胞(图1F-J)上记录到的微小抑制性突触后电流的频率,且该作用具有浓度依赖性(图1K)。结果提示,胰高血糖素样肽1能够增强至视网膜神经节细胞的γ-氨基丁酸的自发释放。
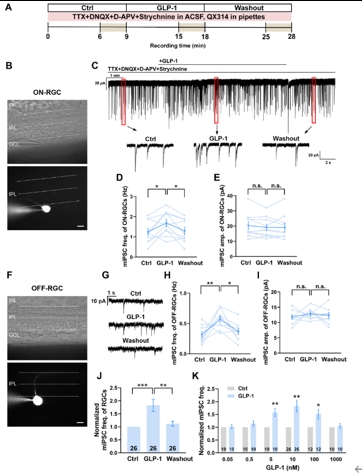
图1胰高血糖素样肽1剂量依赖性地增强视网膜神经节细胞上记录到的微小抑制性突触后电流(图源:Shao et al., Neural Regen Res, 2026)
随后,钟咏梅等通过腹腔注射链脲佐菌素的方法特异性杀死胰岛β细胞,建立Ⅰ型糖尿病大鼠模型。与对照动物相比,糖尿病4周后,视网膜神经节细胞上记录到的微小抑制性突触后电流频率显著降低,而急性灌流胰高血糖素样肽1对糖尿病视网膜神经节细胞上记录到的微小抑制性突触后电流也有明显的增强作用(图2A-D)。进一步采用滴眼的方式给予胰高血糖素样肽1排除全身给药引起的降血糖作用对实验结果的影响。在糖尿病成模2周时开始给予胰高血糖素样肽1滴眼液(2 mg/kg/day),持续2周后(图2E),滴眼胰高血糖素样肽1的大鼠视网膜神经节细胞的微小抑制性突触后电流频率显著高于对照组(图2F-H)。这些结果提示,胰高血糖素样肽1能阻止高血糖诱发的视网膜神经节细胞突触前γ-氨基丁酸释放的减少。
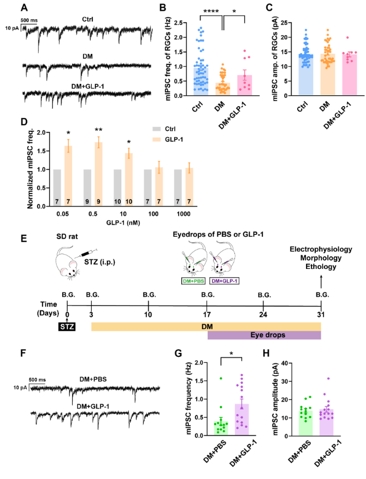
图2胰高血糖素样肽1逆转高血糖诱导的视网膜神经节细胞上微小抑制性突触后电流频率的降低(图源:Shao et al., Neural Regen Res, 2026)
为了确定胰高血糖素样肽1对γ-氨基丁酸系统的调控作用是否能促进糖尿病视网膜神经节细胞的存活,钟咏梅等使用抗Brn3a抗体特异性标记视网膜神经节细胞,并进行细胞计数。结果显示,糖尿病4周后,大鼠视网膜外周区域的视网膜神经节细胞密度显著下降,滴眼胰高血糖素样肽1可使视网膜神经节细胞密度维持在接近对照组水平;而在滴眼胰高血糖素样肽1的同时滴眼给予胰高血糖素样肽1受体拮抗剂Ex-9-39或γ-氨基丁酸A型受体特异性拮抗剂SR95531可取消胰高血糖素样肽1对糖尿病大鼠视网膜神经节细胞的保护作用。与之不同的是,在糖尿病大鼠的视网膜中央区域,作者并未检测到视网膜神经节细胞密度的降低(图3)。这些结果表明,在糖尿病早期,视网膜视网膜神经节细胞由外周向中央呈现渐进式的损伤,而胰高血糖素样肽1通过激活胰高血糖素样肽1受体,增强视网膜神经节细胞上由γ-氨基丁酸A受体介导的抑制性输入,抑制兴奋性毒性过程,从而促进视网膜神经节细胞的存活。
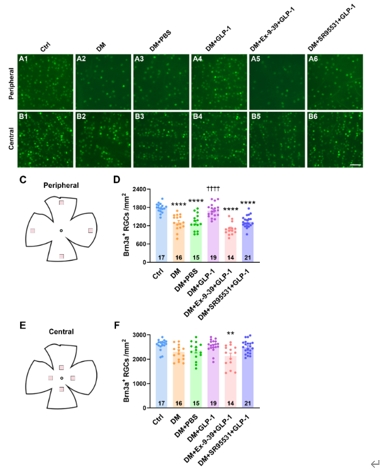
图3滴眼局部给予胰高血糖素样肽1可促进糖尿病视网膜神经节细胞的存活(图源:Shao et al., Neural Regen Res, 2026)
在进一步的功能研究中,钟咏梅等使用多通道微电极阵列技术,记录了视网膜神经节细胞的自发动作电位发放频率以及光诱发的动作电位最大发放频率,作为其光反应功能的体现(图4A)。结果显示,糖尿病4周后,ON型视网膜神经节细胞的自发动作电位发放频率显著增加(图4B和C),而光诱发的动作电位最大发放频率显著降低(图4F和H)。这意味着单个ON型视网膜神经节细胞能够处理和编码的光信号范围变窄,从而影响其向高级视觉中枢的信号输入。与之不同的是,在糖尿病4周这一时间节点,OFF型视网膜神经节细胞的自发动作电位发放频率以及光诱发的动作电位最大发放频率均未受到显著影响(图4D,E,G和I)。在滴眼胰高血糖素样肽1后,糖尿病大鼠ON型视网膜神经节细胞的自发动作电位发放频率与光诱发的动作电位最大发放频率均能维持在对照水平,提示滴眼胰高血糖素样肽1治疗对于糖尿病状态下视网膜神经节细胞的光反应功能具有显著的保护作用。
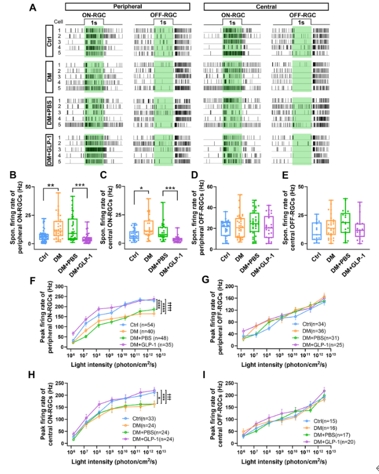
图4滴眼胰高血糖素样肽1增强ON型视网膜神经节细胞的光反应(图源:Shao et al., Neural Regen Res, 2026)
最后,钟咏梅等使用视动行为学检测系统(图5A)测量大鼠的空间分辨率以及视觉敏感度,作为视觉整体功能的反映。4周的糖尿病会导致大鼠的空间分辨率以及视觉敏感度水平显著降低,而滴眼给予胰高血糖素样肽1治疗能够使空间分辨率维持在接近对照组水平(图5B),并能部分地改善糖尿病造成的对比敏感度损伤(图5C)。结果提示,滴眼胰高血糖素样肽1能够显著改善糖尿病早期的视觉功能损伤。
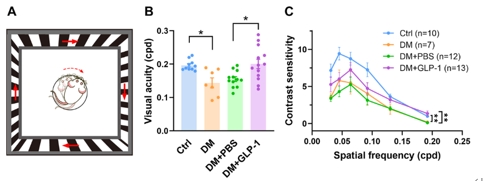
图5胰高血糖素样肽1治疗改善了糖尿病引起的视觉功能损伤(图源:Shao et al., Neural Regen Res, 2026)
此外,钟咏梅等还使用膜片钳技术与药理学手段相结合的方法,详细研究了胰高血糖素样肽1增强视网膜神经节细胞上微小抑制性突触后电流的胞内机制。通过应用不同抑制剂分别阻断磷酸肌醇-磷脂酶C(PI-PLC)、三磷酸肌醇受体(IP3R)介导的胞内钙库释放或蛋白激酶C后,胰高血糖素样肽1不再能增强视网膜神经节细胞上记录到的微小抑制性突触后电流,而施加蛋白激酶C的激动剂可模拟胰高血糖素样肽1的作用,提示胰高血糖素样肽1受体/磷酸肌醇-磷脂酶C/三磷酸肌醇受体/Ca2+/蛋白激酶C信号通路介导了胰高血糖素样肽1对视网膜神经节细胞上微小抑制性突触后电流的增强作用。
该研究表明,胰高血糖素样肽1滴眼液可阻止4周高血糖诱导的视网膜神经节细胞的γ-氨基丁酸自发释放的减少,从而抑制兴奋性毒性过程,促进视网膜神经节细胞的存活。胰高血糖素样肽1受体/磷酸肌醇-磷脂酶C/三磷酸肌醇受体/Ca2+/蛋白激酶C信号通路介导了胰高血糖素样肽1对视网膜神经节细胞上微小抑制性突触后电流的增强作用。同时,滴眼胰高血糖素样肽1还能增强视网膜神经节细胞的光反应,并且改善糖尿病早期的视觉功能损伤。但是研究没有进一步研究胰高血糖素样肽1滴眼后改善糖尿病大鼠视网膜神经节细胞光反应以及视觉整体功能的机制。
综上所述,这项工作揭示内层视网膜γ-氨基丁酸系统的改变在糖尿病早期阶段介导了视网膜神经节细胞的损伤,γ-氨基丁酸系统可能成为抑制早期糖尿病性视网膜病变的潜在治疗靶点。该研究不仅丰富了我们对胰高血糖素样肽1在糖尿病性视网膜病变过程中发挥保护作用的机制的认识,也为胰高血糖素样肽1在预防和治疗糖尿病性视网膜病变的临床应用上提供了新的重要线索。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-00001
参考文献
[1] Teo ZL, Tham YC, Yu M, et al. Global Prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128(11):1580-1591.
[2] Kern TS, Barber AJ. Retinal ganglion cells in diabetes. J Physiol. 2008;586(18):4401-4408.
[3] Zhang Y, Zhang J, Wang Q, et al. Intravitreal injection of exendin-4 analogue protects retinal cells in early diabetic rats. Invest Ophthalmol Vis Sci. 2011;52(1):278-285.
[4] Hernández C, Bogdanov P, Corraliza L, et al. Topical administration of glp-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172-187.
[5] Fan Y, Liu K, Wang Q, et al. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp Eye Res. 2014;127:104-116.




