中国神经再生研究(英文版) ›› 2026, Vol. 21 ›› Issue (2): 742-755.doi: 10.4103/NRR.NRR-D-24-01269
心脏骤停猪模型海马血脑屏障破坏和神经炎症:单细胞RNA测序
Blood–brain barrier disruption and neuroinflammation in the hippocampus of a cardiac arrest porcine model: Single-cell RNA sequencing analysis
Tangxing Jiang1, 2 , 3, 4, 5, Yaning Li1, 2, 3, 4, 5, Hehui Liu1, 2, 3, 4, 5, Yijun Sun1, 2, 3, 4, 5, Huidan Zhang1, 2, 3, 4, 5, Qirui Zhang1, 2, 3, 4, 5, Shuyao Tang1, 2, 3, 4, 5, Xu Niu1, 2, 3, 4, 5, Han Du1, 2, 3, 4, 5, Yinxia Yu1, 2, 3, 4, 5, Hongwei Yue1, 2, 3, 4, 5, Yunyun Guo1, 2, 3, 4, 5, *, Yuguo Chen1, 2, 3, 4, 5, *, Feng Xu1, 2, 3, 4, 5, *
- 1 Department of Emergency Medicine, Qilu Hospital of Shandong University, Jinan, Shandong Province, China; 2 Shandong Provincial Clinical Research Center for Emergency and Critical Care Medicine, Institute of Emergency and Critical Care Medicine of Shandong University, Chest Pain Center, Qilu Hospital of Shandong University, Jinan, Shandong Province, China; 3 Medical and Pharmaceutical Basic Research Innovation Center of Emergency and Critical Care Medicine, China’s Ministry of Education, Shandong Provincial Engineering Laboratory for Emergency and Critical Care Medicine, Key Laboratory of Emergency and Critical Care Medicine of Shandong Province, Key Laboratory of Cardiopulmonary-Cerebral Resuscitation Research of Shandong Province, Qilu Hospital of Shandong University, Jinan, Shandong Province, China; 4 NMPA Key Laboratory for Clinical Research and Evaluation of Innovative Drug, Qilu Hospital of Shandong University, Jinan, Shandong Province, China; 5 National Key Laboratory for Innovation and Transformation of Luobing Theory; The Key Laboratory of Cardiovascular Remodeling and Function Research, Chinese Ministry of Education, Chinese National Health Commission and Chinese Academy of Medical Sciences, Qilu Hospital of Shandong University, Jinan, Shandong Province, China
摘要:
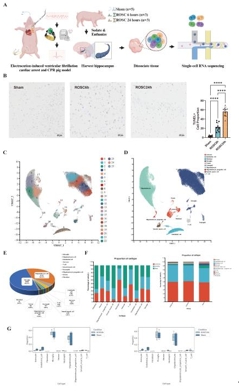
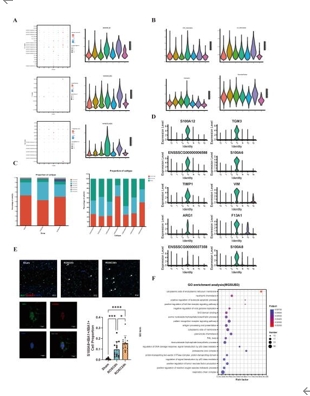
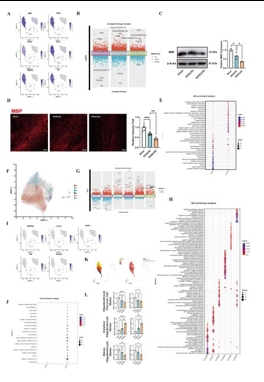
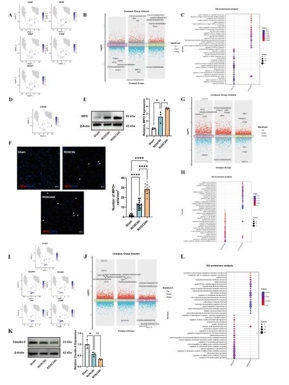
心脏骤停(Cardiac Arrest, CA)可导致全脑缺血和严重的神经功能障碍。尽管复苏科学取得了进步,但由于对心脏骤停后脑损伤的细胞和分子机制了解有限,有效的神经保护策略仍然难以实现。既往有关骤停后脑损伤的研究多集中于神经元死亡,而对非神经元细胞的作用及细胞间通讯的研究不足。为了填补这些空白,实验假设单细胞转录组分析可以发现以前未发现的细胞亚群、改变的细胞通讯网络以及涉及心脏骤停后脑损伤的新分子机制。实验对室颤诱导的心脏骤停模型猪在恢复自主循环后 6和 24 h的海马以及假手术猪进行了单细胞转录组测序研究。心脏骤停后,海马区的细胞组成发生了显著变化。与Sham组相比,ROSC6h组和ROSC24h组中星形胶质细胞和内皮细胞的比例显著减少,而小胶质细胞、中性粒细胞和T细胞的比例则显著增加。这些变化不仅表明血脑屏障功能受损,还提示外周免疫细胞通过受损的血脑屏障浸润到脑实质中,从而加剧神经炎症。实验进一步鉴定并验证了一个独特的高表达 S100A8 的活化小胶质细胞亚群,其丰度在脑梗死后随着时间的推移而增加。该亚群同时表现出明显的 M1/M2 极化,并表达与趋化因子和白介素相关的关键功能基因。此外,还发现了少突胶质细胞在脑损伤后的功能障碍以及少突胶质细胞前体细胞向少突胶质细胞的分化。通过细胞通讯分析发现心脏骤停后嗜中性粒细胞与小胶质细胞之间的通讯增强了,这是由嗜中性粒细胞来源抵抗素介导的,它推动了促炎性小胶质细胞极化。此实验提供了心脏骤停后海马的全面单细胞图谱,为心脏骤停后的神经保护和修复提供了潜在的新靶点。
https://orcid.org/0000-0002-1664-9125 (Yunyun Guo); https://orcid.org/0000-0001-9501-2546 (Yuguo Chen);
https://orcid.org/0000-0002-4670-3727 (Feng Xu)