中国神经再生研究(英文版) ›› 2023, Vol. 18 ›› Issue (10): 2237-2245.doi: 10.4103/1673-5374.369115
Hoxa5启动子组蛋白乙酰化修饰抑制急性缺血性脑卒中神经元凋亡
-
出版日期:2023-10-15发布日期:2023-03-28 -
基金资助:辽宁省自然科学基金项目(2021-MS-061)
The circular RNA Rap1b promotes Hoxa5 transcription by recruiting Kat7 and leading to increased Fam3a expression, which inhibits neuronal apoptosis in acute ischemic stroke
Fang-Fang Zhang#, Liang Zhang*, #, Lin Zhao#, Yu Lu, Xin Dong, Yan-Qi Liu, Yu Li, Shuang Guo, Si-Yuan Zheng, Ying Xiao, Yu-Zhu Jiang
- Department of Rehabilitation Medicine, The People’s Hospital of China Medical University (The People’s Hospital of Liaoning Province), Shenyang, Liaoning Province, China
-
Online:2023-10-15Published:2023-03-28 -
Contact:Liang Zhang, MS, bafeng4242@163.com or 464473517@qq.com. -
Supported by:This work was supported by the Natural Science Foundation of Liaoning Province, No. 2021-MS-061 (to LZhang)
摘要:
环状RNA可参与调控缺血性脑血管病的发生和进展,但是是否也对急性缺血性脑卒中有作用,目前尚不可知。为探索circRap1b对急性缺血性脑卒中的作用,实验分别建立了氧糖剥夺HT22细胞建立体外急性缺血缺氧模型以及右侧颈动脉闭塞急性缺血缺氧小鼠模型。结果发现,circRap1b在急性缺血缺氧小鼠海马以及HT22细胞中均显著下调,而过表达circR ap1b能有效抑制急性缺血缺氧损伤HT22细胞的凋亡。同时circRap1b/Hoxa5在体外抑制神经元凋亡可能归因于Kat7诱导的Hoxa5启动子区组蛋白H3赖氨酸14乙酰化修饰。这一结果说明了环状RNA Rap1b抑制急性缺血性卒中神经元凋亡的机制。
https://orcid.org/0000-0003-0604-8307 (Liang Zhang)
引用本文
. Hoxa5启动子组蛋白乙酰化修饰抑制急性缺血性脑卒中神经元凋亡[J]. 中国神经再生研究(英文版), 2023, 18(10): 2237-2245.
Fang-Fang Zhang, Liang Zhang, Lin Zhao, Yu Lu, Xin Dong, Yan-Qi Liu, Yu Li, Shuang Guo, Si-Yuan Zheng, Ying Xiao, Yu-Zhu Jiang. The circular RNA Rap1b promotes Hoxa5 transcription by recruiting Kat7 and leading to increased Fam3a expression, which inhibits neuronal apoptosis in acute ischemic stroke[J]. Neural Regeneration Research, 2023, 18(10): 2237-2245.
| [1] | . 血清反应因子可促进脊髓横断后的轴突再生[J]. 中国神经再生研究(英文版), 2023, 18(9): 1956-1960. |
| [2] | . CXC趋化因子受体7增强缺血性脑卒中后的神经可塑性[J]. 中国神经再生研究(英文版), 2023, 18(9): 1976-1982. |
| [3] | . miR-181b能促进缺血性脑卒中后血管生成和神经功能的恢复[J]. 中国神经再生研究(英文版), 2023, 18(9): 1983-1989. |
| [4] | . 光生物调节对脊髓损伤的神经保护:调节亚急性期线粒体动力学失衡[J]. 中国神经再生研究(英文版), 2023, 18(9): 2005-2010. |
| [5] | . 抑制5-羟色胺受体3可缓解阿尔茨海默病小鼠模型的病理变化[J]. 中国神经再生研究(英文版), 2023, 18(9): 2019-2028. |
| [6] | . 与核受体相关因子1缺陷导致帕金森病多巴胺能神经元损伤的分子机制:单核RNA测序分析[J]. 中国神经再生研究(英文版), 2023, 18(9): 2037-2046. |
| [7] | . 5-羟色胺:肌萎缩侧索硬化的潜在干预靶点[J]. 中国神经再生研究(英文版), 2023, 18(9): 2047-2055. |
| [8] | . 背根神经节神经元轴突再生过程中的基因表达谱:联合激光显微切割和深度测序鉴定[J]. 中国神经再生研究(英文版), 2023, 18(9): 2056-2066. |
| [9] | . 减轻吗啡耐受:抑制高迁移率族蛋白B1释放或AMP依赖的蛋白激酶-血红素加氧酶1信号通路[J]. 中国神经再生研究(英文版), 2023, 18(9): 2067-2074. |
| [10] | . 敲低NADPH氧化酶4可减轻脑出血后线粒体氧化应激和神经元焦亡[J]. 中国神经再生研究(英文版), 2023, 18(8): 1734-1742. |
| [11] | . 内皮细胞丝裂原活化蛋白激酶磷酸酶1活化能保护改善缺血性脑卒中血脑屏障[J]. 中国神经再生研究(英文版), 2023, 18(8): 1743-1749. |
| [12] | . 抑制压电型机械敏感离子通道组件1可抑制脑出血后的脱髓鞘[J]. 中国神经再生研究(英文版), 2023, 18(8): 1750-1756. |
| [13] | . 脑卒中后亚急性期抑制Notch信号可促进纹状体星形胶质细胞转化为神经元[J]. 中国神经再生研究(英文版), 2023, 18(8): 1777-1781. |
| [14] | . 聚(ADP-核糖)聚合酶成员14沉默不利于脊髓损伤后的功能恢复[J]. 中国神经再生研究(英文版), 2023, 18(8): 1809-1817. |
| [15] | . 基于狂犬病病毒的高效逆行转导和逆行跨单突触示踪的工具病毒系统[J]. 中国神经再生研究(英文版), 2023, 18(8): 1827-1833. |
出版重点
《中国神经再生研究(英文版)》杂志为SCI、PubMed数据库收录的国际唯一一本专注神经再生领域研究的经同行评议的开放获取期刊,出版来自全球神经再生领域专业学者的前沿性基础研究及临床研究及转化医学、循证医学优秀的最新成果。
期刊出版来自于脑损伤与神经再生、脊髓损伤与神经再生、周围神经损伤与神经再生和神经退行性病与神经再生、神经影像与神经再生的相关研究。期刊关注神经损伤与再生过程中的轴突再生、突触生长、神经可塑性、神经修复和替代、神经移植等最新研究成果。尤其关注应用细胞治疗、基因治疗、生物因子治疗、药物治疗、手术治疗、康复治疗、物理疗法、组织工程、生物工程、生物材料、神经假体等干预性方法产生神经再生效果的相关研究。文章应清晰描述抑制神经元损伤、减轻神经元损伤的一系列变化,保护损伤神经元的过程、方法、程度与评价,突出从细胞分子水平以及分子生物学水平解释神经元损伤后以及预后再生的机制。
NRR杂志被国际重要数据库收录
科学引文索引(Science Citation Index Expanded,SCI)
美国国立医学图书馆(PubMed)
美国国立医学图书馆开放获取全文数据库(PubMed Central, PMC)
美国生物学文摘数据库(BIOSIS previews, BP)
美国《化学文摘》(Chemical Abstracts, CA)
Scopus荷兰《医学文摘库/医学文摘》(Excerpta Medica, EM)
波兰《哥伯尼索引》(Index of Copurnicus, IC)
OvidSP平台数据库
中国科学引文数据库(CSCD)
中国科技期刊数据库-统计源期刊(CSTPCD)
编委会
主编Editor-in-Chief
苏国辉院士(Kwok-fai So, Chair Professor and Head, Jessie Ho Professor in Neuroscience, Department of Anatomy, The University of Hong Kong)。
徐晓明教授(Xiao-ming Xu, Professor and Mari Hulman George Chair of Neurological Surgery, Scientific Director of Spinal Cord and Brain Injury Research Group, Indiana University School of Medicine)
联系方式:Email: szb@nrren.org 电话:+86 138 0499 8773
编委会成员
期刊编委队伍由国际神经再生领域著名学者、中国科学院院士、香港大学苏国辉教授和美国印第安纳大学徐晓明教授领导的由100多位国际神经再生优秀专家组成。共同致力于创办一本发表神经再生领域专业学术研究经同行严格评审的优秀学术期刊。
在线投稿平台
作者可以通过www.nrronline.org在线投稿。
所有的稿件都将通过该系统提交至NRR杂志电子投稿出版管理系统。
作者有不明确的问题,请访问szb@nrren.org或咨询+86 138 0499 8773。
投稿后,当论文已处于审稿或等待审稿状态时,2个月内请勿将稿件再投至他刊。
初次投稿:
投稿信:
应说明文章未一稿多投,全部作者是否对所投稿件内容知情同意,推荐2-3位小同行审稿人。
作者协议:
投稿时请注意作者协议,如同意后可继续完成投稿, 投稿成功后作者已与杂志签定了文章的相关版权。
投稿后的同行评议
期刊投稿平台应用国际最大的投稿平台Editorial Manager,投到本刊的每篇稿件都要经过3-4位小同行审稿人评审,审稿方法为国际学科小同行组成双盲审稿。所有发表在杂志的文章都将经过严格的双盲同行层层评议,审稿中注重科学性、伦理学和文章真实性的严格审查。期刊将在投稿后4周内通知作者评审意见。
根据评审意见,编辑部决定稿件返修,再审,被接受或退稿。 稿件被接受后,作者可经投稿平台以通讯作者账号随时查询稿件的出版进程。
出版时间
一般稿件被采用后3-4个月出版,优秀文章可在被采用后1-2个月内发表,有临床试验注册号的优秀临床试验文章可申请加急发表。
出版后传播
文章出版后将在EurekAlert!和EurekAlert!中文新闻平台以中英文双语形式向全世界同仁传播。EurekAlert!和EurekAlert!中文新闻平台是美国科学促进会(AAAS)主办的一项全球的科学新闻服务。美国科学促进会为世界最大的科学协会,并且是《科学》杂志的出版机构。您的文章将以最快时间推送给经严格验证的来自全球的新闻记者8000余人,包括来自国际的纽约时报、华盛顿邮报和路透社等,来自中国的中国日报、新华社、人民日报等国际主流媒体,为科学家们提供了与国际科学记者和国际学术平台直接沟通的一个快速有效的桥梁。我们将随后为您提供您新闻的网站点击情况和媒体报告情况的具体数据。
出版后的新闻传播将极大提高文章的影响力,统计同时表明发布学术新闻的文章将提高其被引率70%以上。
杂志的读者群Audience
来自全球从事神经再生、神经科学、神经解剖、神经病理、神经外科、神经内科、神经生物、神经影像、神经放射、神经康复等领域的学科专家。
对特邀综述稿件的要求:
(1)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(2)全文不超过6000个单词,包括摘要,不包括参考文献,图和表格 ,出版后8-10个版面。
(3)文章写作结构:
文题:不超过 90个字母,20个单词。
摘要:非结构式, 250单词。
引言:
主体内容:
总结:
作者贡献:
利益冲突:
参考文献:采用 Journal of Neuroscience格式。
特邀述评类文章:观点、点评 、研究亮点、给编辑的信等。
特邀观点栏目文章要求:
(1)观点文章为作者对神经再生领域某一热点问题 的评论,有作者鲜明的观点和作者本人
对此科研过程的认识 和总结。
(2)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(3)全文2000- 3000单词,包括参考文献,不需要图和表格,不需要摘要,出 版后2个版面。
(4)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
特邀点评与研究亮点栏目文章 要求:
(1)点评文章为点评在本刊发表的文章,研究亮点 文章为点评国际优秀杂志近期或在线提前发表的前沿性的优秀文章。
(2)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(3)全文2000- 3000单词,包括参考文献,不需要图和表格,不需要摘要,出 版后2个版面。
(4)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
利益冲突:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
给编辑的信栏目文章要求:
(1)给编辑的信文章为读者对本刊已发表文章的来 信反馈。
(2)全文1000- 2000单词,不包括参考文献,文章不需要摘要、图和表格,出 版后1个版面。
(4)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
利益冲突:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
如果您需要向SCI收录期刊投稿,我们可以为您提供如下服务--
NRR:辽宁省人民医院张亮团队揭示环状RNA Rap1b抑制急性缺血性卒中神经元凋亡的机制
撰写:张芳芳、赵琳、陆宇、董欣、张亮
脑卒中是一种常见的神经系统疾病,是全球范围内永久性发病和致残的主要原因之一[1, 2]。脑卒中的2种主要类型是急性缺血性脑卒中(acute ischemic stroke,AIS)和出血性脑卒中。目前美国食品药品监督管理局批准的急性缺血性脑卒中治疗方法只有在发病后4-5 h内静脉注射重组人组织型纤溶酶原激活剂,但由于其治疗时间窗狭窄,且存在颅内出血和血管性水肿的风险,只有约7%的患者符合治疗条件,因此对缺血性卒中的溶栓治疗效果有限[3-7]。急性缺血性脑卒中后,动脉供血受阻的急性缺血初级神经元会迅速死亡,而由其他动脉输入的核心和周围的急性缺血初级神经元则在一定程度上存在延迟凋亡的现象[8, 9]。虽然多种药物在动物模型中表现出明显的神经保护作用,但这些结果并未得到临床试验的证实[10]。鉴于缺血性脑损伤及其全身后果的复杂性,通过靶向多种机制的细胞疗法可能比单一药物治疗更为有效[11, 12]。环状RNA (circular RNA,circRNA)是一类新型非编码RNA,为单链、稳定的RNA,其在3'端和5'端之间存在共价键,可形成无多聚腺苷化尾部的环状结构,因而不受RNA外切酶的影响[13-15]。最近有研究表明,环状RNA高度保守,且在人类细胞中大量表达[13, 16, 17]。最新研究发现,环状RNA的表达具有组织特异性,并受不同的生物学过程调节,如卒中、神经炎症和衰老、心肌肥厚和心力衰竭以及细胞生长[18-23]。微小RNA靶向Rap1b可抑制多种肿瘤类型的细胞迁移、侵袭和转移[24-28]。然而,Rap1b在神经系统疾病中的功能作用尚不清楚,研究者旨在探索circRap1b在急性缺血性脑卒中中的作用。
近期,来自中国辽宁省人民医院张亮团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“The circular RNA Rap1b promotes Hoxa5 transcription by recruiting Kat7 and leading to increased Fam3a expression, which inhibits neuronal apoptosis in acute ischemic stroke”的研究中发现,circRap1b在急性缺血性脑卒中C57BL/6J小鼠海马组织和神经元HT22细胞中表达下调。过表达circRap1b可抑制急性缺血性脑卒中体外模型HT22细胞的凋亡。而circRap1b/Hoxa5在体外抑制神经元凋亡可能归因于Kat7诱导的Hoxa5启动子区组蛋白H3赖氨酸14乙酰化修饰,这一结果也在体内实验中得到验证。这一研究启示,circRap1b有望成为脑卒中治疗的新靶点。
研究表明,环状RNA可参与调控缺血性脑血管病的发生和进展[23, 29, 30]。circ DLGAP4通过结合miR-143调控HER1表达,影响内皮细胞去分化为间充质细胞,从而减轻脑梗死体积和血脑屏障损伤。环状RNA还能参与调节神经元等多种细胞的凋亡。circ_0000950在阿尔茨海默病发展过程中可通过miR-103负调控神经元凋亡[31]。此外,circ_0000296在慢性脑缺血缺氧海马组织和神经元HT22细胞中表达下调,且过表达circ_0000296能显著抑制慢性脑缺血缺氧诱导的神经元凋亡[32]。张亮等发现,C57BL/6J小鼠和神经元HT22细胞经急性缺血缺氧后,苏木精-伊红染色和TUNEL染色可见神经元凋亡率明显增加(图1A-C)。定量聚合酶链式反应发现,circRap1b在急性缺血缺氧损伤的小鼠海马和HT22细胞中低表达(图1D-G)。而circRap1b过表达显著抑制急性缺血缺氧诱导的HT22神经元凋亡(图1J-K)。
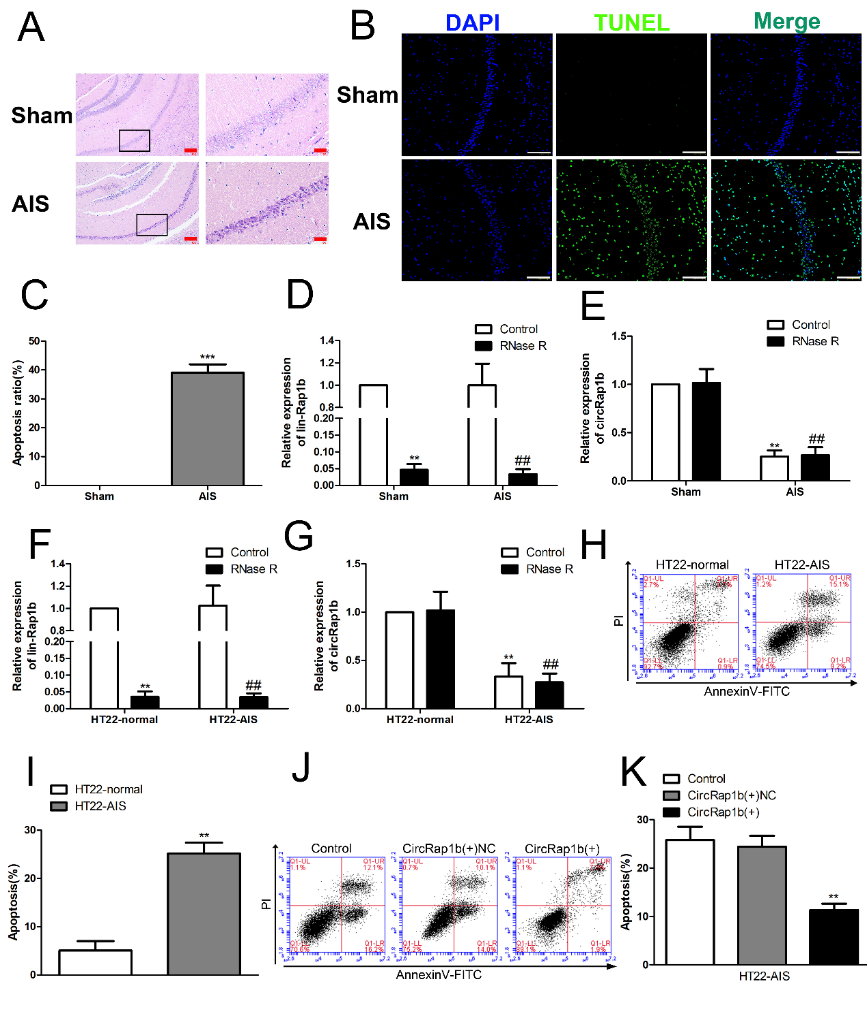
图1 CircRap1b在急性缺血缺氧损伤小鼠海马以及HT22细胞中低表达,且可抑制神经元凋亡(图源:Zhang et al., Neural Regen Res, 2023)
在circRap1b过表达引起的差异表达蛋白中,转录因子Hoxa5表达上调最明显(图2)。Hox基因家族编码的转录因子有助于决定神经元的命运[18]。具体来说,Hoxa5最近被证明参与了小鼠大脑中具有自主功能的核细胞[33]和后迷走神经运动神经元[29]的轴突生长。Hernández等[34]提出,Hoxa5是人脂肪来源干细胞分化为神经元的重要基因,因此是探索神经疾病细胞治疗策略的潜在候选基因。研究显示,Hoxa5在急性缺血缺氧小鼠海马和HT22细胞中低表达(图3A-E)。流式细胞仪检测发现过表达Hoxa5显著抑制了急性缺血缺氧诱导的神经元凋亡,而敲低Hoxa5后HT22细胞的凋亡率明显恢复(图3F和G)。研究显示,过表达增生性瘢痕或瘢痕疙瘩来源的成纤维细胞中的HOXA5减弱了细胞增殖、迁移和胶原合成,而加速了细胞凋亡[35]。沉默HOXA5基因表达可诱导Jurkat细胞凋亡和细胞周期阻滞[36]。且HOXA5直接作用于维甲酸受体的下游,参与维甲酸诱导的细胞凋亡和生长抑制[37]。实验显示,Hoxa13在慢性脑缺血缺氧诱导的海马和HT22细胞中表达下调,而过表达Hoxa13减弱了慢性脑缺血缺氧诱导的海马神经元HT22的凋亡[38]。同时小鼠Hoxa13突变体通过下调Bmp7表达抑制尿路上皮细胞凋亡[39]。
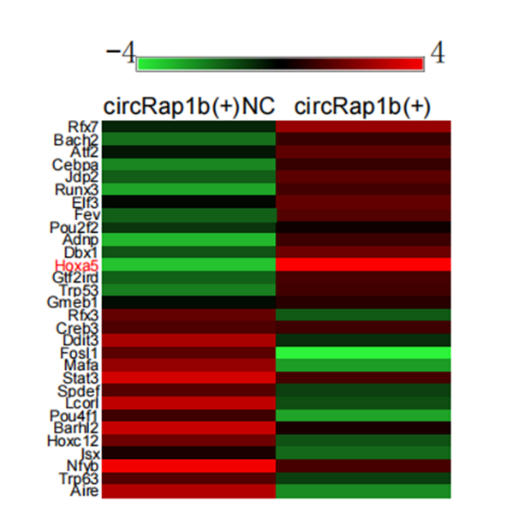
图2 在circRap1b过表达引起的差异表达蛋白中,转录因子Hoxa5表达上调最明显(图源:Zhang et al., Neural Regen Res, 2023)
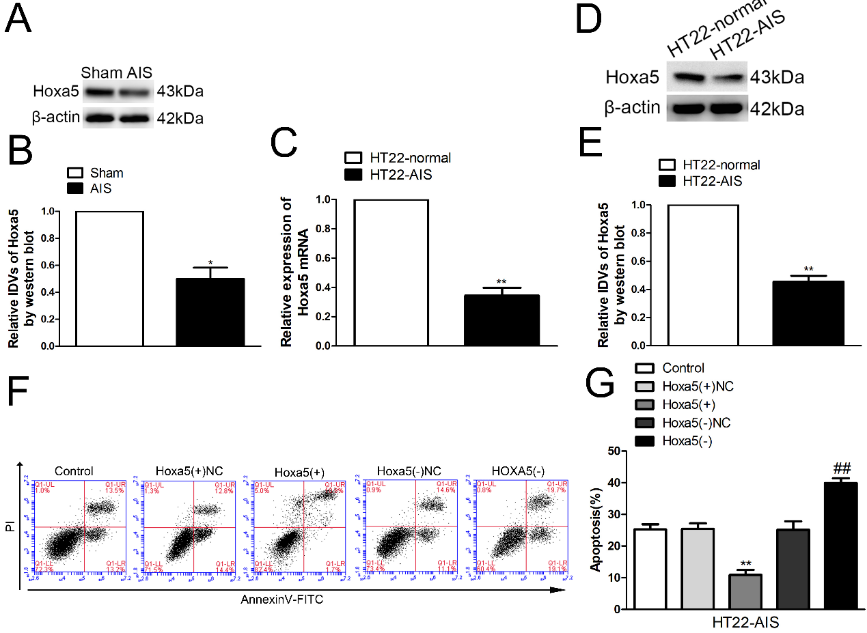
图3 Hoxa5在急性缺血缺氧海马和HT22细胞中低表达,并可抑制神经元的凋亡(图源:Zhang et al., Neural Regen Res, 2023)
组蛋白修饰主要包括甲基化、乙酰化、磷酸化、泛素化以及类泛素化[40]。组蛋白乙酰化受组蛋白乙酰基转移酶和组蛋白去乙酰化酶的动态调控。既往研究表明,Kat7倾向于催化组蛋白H4的赖氨酸5和赖氨酸12以及组蛋白H3的赖氨酸14的乙酰化[41, 42]。有研究发现,过表达circFoxo3在体外抑制氧糖剥夺并诱导心肌细胞自噬、凋亡、炎症和损伤。过表达circFoxo3可通过减少KAT7、H3K14ac和RNA Poly II在HMGB1启动子中的富集而抑制HMGB1表达,从而抑制自噬,减轻心肌缺血再灌注损伤[43]。在哺乳动物细胞中过表达CDK11(P58)可增强Kat7对游离组蛋白的乙酰转移酶活性。CDK11(P58)是一种Kat7的调节蛋白,可在体内外与Kat7相互作用,在细胞周期进程中发挥重要作用,并与凋亡密切相关[44]。张亮等过表达circRap1b后,Hoxa5 mRNA和蛋白表达显著增加(图4A)。荧光原位杂交技术结果显示,circRap1b存在于细胞核(图4B),与Kat7相互作用共存于Hoxa5的启动子区(图4C-E)。H3K14ac在Hoxa5启动子区富集,主要富集与-1000-0 bp(图4F和G)。在急性缺血缺氧HT22细胞敲低Kat7可逆转circRap1b过表达对H3K14ac富集和Hoxa5 mRNA和蛋白表达的影响(图4H-L)。进一步RNA下拉和染色质免疫共沉淀结果同样证实H3K14ac可与circRap1b相互作用,且circRap1b存在于Hoxa5启动子区(图4M-O)。因此实验结果说明,circRap1b可招募Kat7诱导Hoxa5启动子区H3K14ac修饰来激活其转录。既往也有研究显示,circMRPS35可通过募集KAT7升高FOXO1和FOXO3a启动子区H4K5ac水平,从而抑制胃癌进展[45]。与本次研究的结果相似,lncRNA-MRCCAT1可通过结合EZH2来诱导NPR3启动子区域的H3K27me3来介导NPR3转录[46]。将MALAT1与PRC2结合,分离EZH2与HIV-1 LTR启动子的结合,去除H3K27me3,可逆转人类免疫缺陷病毒转录的表观遗传沉默[47]。

图4 circRap1b可通过招募乙酰基转移酶Kat7诱导Hoxa5启动子区H3K14ac修饰,促进Hoxa5的转录(图源:Zhang et al., Neural Regen Res, 2023)
序列相似家族3A (Family with sequence similarity 3A,FAM3A)是一种线粒体蛋白,可在细胞适应应激和细胞存活中发挥重要作用,并参与神经元凋亡的调节[38, 48]。据报道,序列相似家族3A是内质网应激诱导的HT22细胞死亡的重要调节因子[49]。序列相似家族3A可通过激活PI3K/Akt通路保护HT22细胞免受过氧化氢诱导的氧化应激[50]。序列相似家族3A还在慢性脑缺血缺氧诱导的海马和神经元中表达下调,而过表达序列相似家族3A则可抑制慢性脑缺血缺氧诱导的海马神经元凋亡[38]。张亮等发现序列相似家族3A在急性缺血性脑卒中诱导的海马和HT22中低表达,而过表达序列相似家族3A则显著抑制损伤HT22细胞的凋亡,且敲低序列相似家族3A则促进损伤HT22细胞的凋亡(图5)。据报道,序列相似家族3A可通过激活促生存PI3K/AKT/mTOR通路抑制白细胞介素1β诱导的软骨细胞凋亡[48]。序列相似家族3A可正向调节内皮细胞的缺血后血管生成[51]。序列相似家族3A可减轻新生小鼠的缺氧缺血性脑损伤[52]。根据JASPAR生物信息学数据库,在人序列相似家族3A的启动子区域-3000-0 bp发现了44个HOXA5的推测结合位点,且在小鼠序列相似家族3A的启动子区域-3000-0 bp发现了4个HOXA5的推测结合位点。进一步采用染色质免疫共沉淀和荧光素酶报告基因系统验证Hoxa5可结合序列相似家族3A的启动子区域,促进其转录和蛋白表达(图5),从而调控海马神经元的凋亡。Hoxa13还可通过转录激活序列相似家族3A蛋白的表达,抑制慢性脑缺血缺氧诱导的海马神经元凋亡[38]。Wu等[53]发现Hoxa13通过靶向BMP7启动子区域促进胃癌中的转录表达。Hoxa13靶向Aldh1a2启动子并上调其表达来调节肢体形态发生[54]。
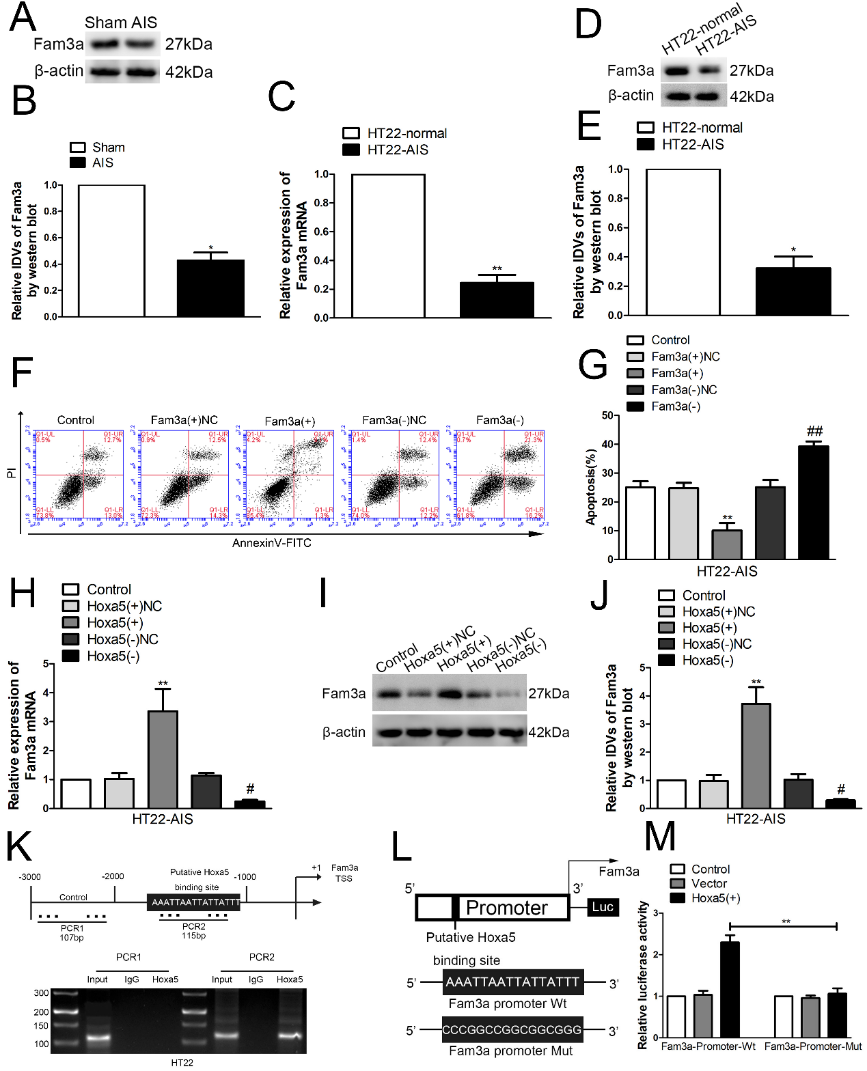
图5 Hoxa5可通过转录激活序列相似家族3A的表达调控神经元凋亡(图源:Zhang et al., Neural Regen Res, 2023)
为进一步阐明circRap1b和Hoxa5共调控急性缺血损伤HT22细胞凋亡的机制,张亮等转染了circRap1b过表达以及Hoxa5的小干扰RNA,流式细胞仪检测发现,过表达circRap1b可抑制细胞凋亡,而敲低Hoxa5则促进细胞凋亡,且敲低Hoxa5可逆转单独circRap1b过表达对细胞凋亡的抑制作用(图6 A和B)。过表达circRap1b后,Hoxa5和序列相似家族3A的mRNA和蛋白表达水平均上调,而敲低Hoxa5则可逆转过表达circRap1b对序列相似家族3A 蛋白的影响。提示circRap1b可通过Hoxa5调控序列相似家族3A的表达(图6C-H)。最后体内实验验证了过表达circRap1b或Hoxa5后,小鼠海马神经元凋亡明显减少,且同时过表达circRap1b和Hoxa5的凋亡率最低(图7A-D)。
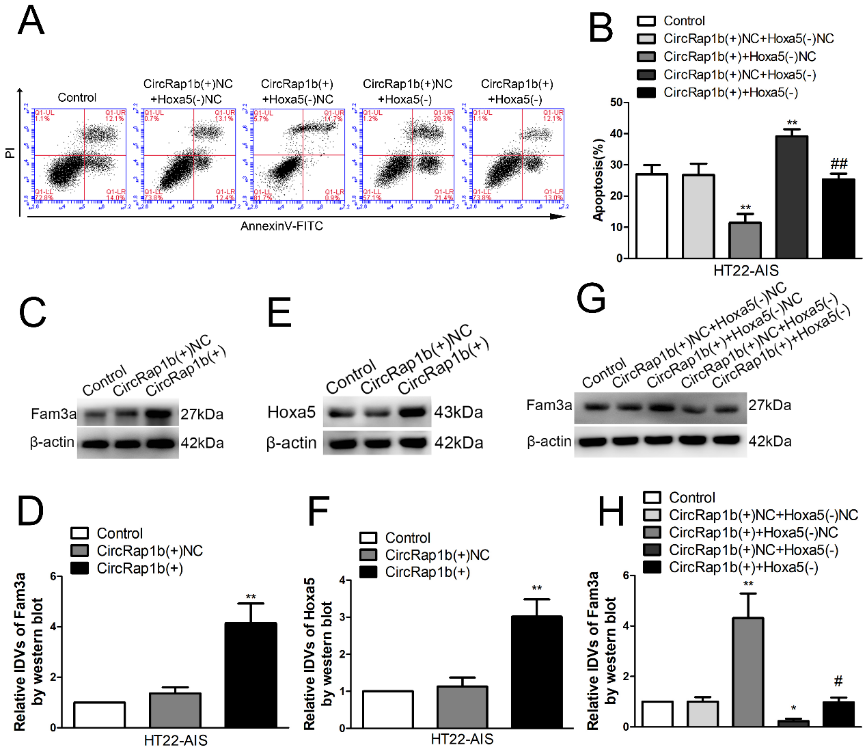
图6 circRap1b可通过Hoxa5调控序列相似家族3A的表达,调节神经元的凋亡(图源:Zhang et al., Neural Regen Res, 2023)
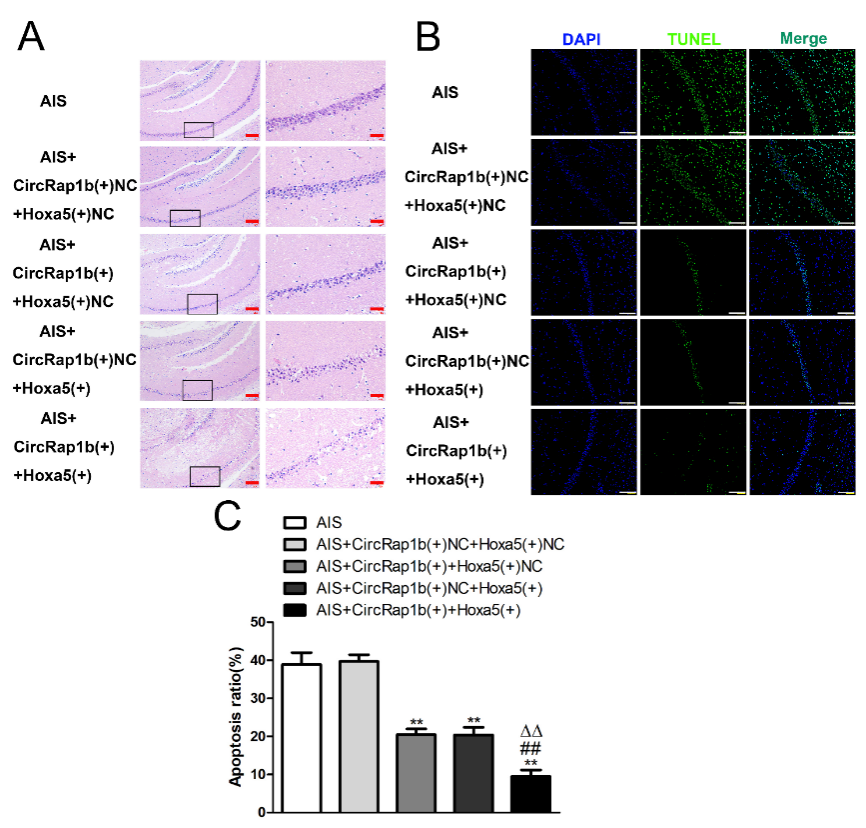
图7 circRap1b和Hoxa5可在体内抑制神经元的凋亡(图源:Zhang et al., Neural Regen Res, 2023)
综上所述,circRap1b可作为模块化支架在Hoxa5的启动子区域招募Kat7,从而提高H3K14ac修饰水平,激活Hoxa5的转录和表达,最终抑制海马神经元的凋亡。目前已有研究利用慢病毒或腺病毒载体在体内过表达天然的保护性环状RNA。这些RNA可编码带有外显子的微盒、内源性剪接供体和受体位点,支持RNA反向折叠的侧翼内含子的反向重复(Holdt等人,2018)。如微量注射circDLGAP4慢病毒可显著改善缺血性卒中预后(Bai et al., 2018)。图8为circRap1b / Hoxa5 /序列相似家族3A轴调控神经元凋亡的机制示意图。研究可能为开发急性缺血性脑卒中的治疗方法和拓宽治疗靶点提供新思路,但研究也存在局限性:首先,该研究只关注雄性大鼠。为了有更多的临床意义,未来的研究应该考虑使用雌性大鼠。其次,尚无转化医学研究。需要加强转化医学研究,开拓脑卒中治疗的新方式。
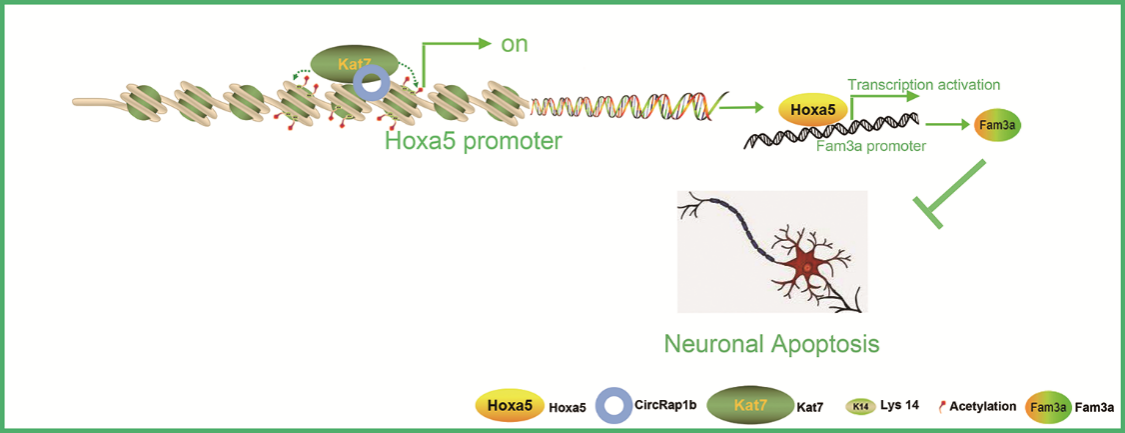
图8 circRap1b/Hoxa5/序列相似家族3A轴调控神经元凋亡(图源:Zhang et al., Neural Regen Res, 2023)
原文链接:https://doi.org/10.4103/1673-5374.369115
参考文献
[1] Spescha RD, Shi Y, Wegener S, et al. Deletion of the ageing gene p66(Shc) reduces early stroke size following ischaemia/reperfusion brain injury. Eur Heart J. 2013;34(2):96-103.
[2] Knowland D, Arac A, Sekiguchi KJ, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82(3):603-617.
[3] Collen D, Lijnen HR. The tissue-type plasminogen activator story. Arterioscler Thromb Vasc Biol. 2009;29(8):1151-1155.
[4] Schwamm LH, Ali SF, Reeves MJ, et al. Temporal trends in patient characteristics and treatment with intravenous thrombolysis among acute ischemic stroke patients at Get With The Guidelines-Stroke hospitals. Circ Cardiovasc Qual Outcomes. 2013;6(5):543-549.
[5] Denorme F, Langhauser F, Desender L, et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood. 2016;127(19):2337-2345.
[6] Snow SJ. Stroke and t-PA--Triggering New Paradigms of Care. N Engl J Med. 2016;374(9):809-811.
[7] Alberts MJ. Stroke Treatment With Intravenous Tissue-Type Plasminogen Activator: More Proof That Time Is Brain. Circulation. 2017;135(2):140-142.
[8] Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331-339.
[9] Fann DY, Lee SY, Manzanero S, et al. Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev. 2013;12(4):941-966.
[10] Wei L, Wei ZZ, Jiang MQ, et al. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol. 2017;157:49-78.
[11] Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8(8):1193-1201.
[12] Vieira MS, Santos AK, Vasconcellos R, et al. Neural stem cell differentiation into mature neurons: Mechanisms of regulation and biotechnological applications. Biotechnol Adv. 2018;36(7):1946-1970.
[13] Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495(7441):333-338.
[14] Vicens Q, Westhof E. Biogenesis of Circular RNAs. Cell. 2014;159(1):13-14.
[15] Li X, Yang L, Chen LL. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol Cell. 2018;71(3):428-442.
[16] Jeck WR, Sorrentino JA, Wang K, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19(2):141-157.
[17] Rybak-Wolf A, Stottmeister C, Glazar P, et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol Cell. 2015;58(5):870-885.
[18] Boeckel JN, Jaé N, Heumüller AW, et al. Identification and Characterization of Hypoxia-Regulated Endothelial Circular RNA. Circ Res. 2015;117(10):884-890.
[19] Wang K, Long B, Liu F, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37(33):2602-2611.
[20] Du WW, Yang W, Chen Y, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38(18):1402-1412.
[21] Huang R, Zhang Y, Han B, et al. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy. 2017;13(10):1722-1741.
[22] Yang L, Han B, Zhang Y, et al. Engagement of circular RNA HECW2 in the nonautophagic role of ATG5 implicated in the endothelial-mesenchymal transition. Autophagy. 2018;14(3):404-418.
[23] Han B, Zhang Y, Zhang Y, et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14(7):1164-1184.
[24] Zhang L, Cui M, Song L, et al. Function, Significance, and Regulation of Rap1b in Malignancy. Crit Rev Eukaryot Gene Expr. 2019;29(2):151-160.
[25] Zhang M, Zhou S, Zhang L, et al. miR-518b is down-regulated, and involved in cell proliferation and invasion by targeting Rap1b in esophageal squamous cell carcinoma. FEBS Lett. 2012;586(19):3508-3521.
[26] Bischoff A, Huck B, Keller B, et al. miR149 functions as a tumor suppressor by controlling breast epithelial cell migration and invasion. Cancer Res. 2014;74(18):5256-5265.
[27] Peng H, Luo J, Hao H, et al. MicroRNA-100 regulates SW620 colorectal cancer cell proliferation and invasion by targeting RAP1B. Oncol Rep. 2014;31(5):2055-2062.
[28] Lin KT, Yeh YM, Chuang CM, et al. Glucocorticoids mediate induction of microRNA-708 to suppress ovarian cancer metastasis through targeting Rap1B. Nat Commun. 2015;6:5917.
[29] Lin SP, Ye S, Long Y, et al. Circular RNA expression alterations are involved in OGD/R-induced neuron injury. Biochem Biophys Res Commun. 2016;471(1):52-56.
[30] Mehta SL, Pandi G, Vemuganti R. Circular RNA Expression Profiles Alter Significantly in Mouse Brain After Transient Focal Ischemia. Stroke. 2017;48(9):2541-2548.
[31] Yang J, Chen M, Cao RY, et al. The Role of Circular RNAs in Cerebral Ischemic Diseases: Ischemic Stroke and Cerebral Ischemia/Reperfusion Injury. Adv Exp Med Biol. 2018;1087:309-325.
[32] Huang K, Yang C, Zheng J, et al. Effect of circular RNA, mmu_circ_0000296, on neuronal apoptosis in chronic cerebral ischaemia via the miR-194-5p/Runx3/Sirt1 axis. Cell Death Discov. 2021;7(1):124.
[33] Salzman J. Circular RNA Expression: Its Potential Regulation and Function. Trends Genet. 2016;32(5):309-316.
[34] Hernández R, Jiménez-Luna C, Ortiz R, et al. Impact of the Epigenetically Regulated Hoxa-5 Gene in Neural Differentiation from Human Adipose-Derived Stem Cells. Biology (Basel). 2021;10(8):802.
[35] Liang Y, Zhou R, Fu X, et al. HOXA5 counteracts the function of pathological scar-derived fibroblasts by partially activating p53 signaling. Cell Death Dis. 2021;12(1):40.
[36] Huang HP, Liu WJ, Guo QL, et al. Effect of silencing HOXA5 gene expression using RNA interference on cell cycle and apoptosis in Jurkat cells. Int J Mol Med. 2016;37(3):669-678.
[37] Chen H, Zhang H, Lee J, et al. HOXA5 acts directly downstream of retinoic acid receptor beta and contributes to retinoic acid-induced apoptosis and growth inhibition. Cancer Res. 2007;67(17):8007-8013.
[38] Liu J, An P, Xue Y, et al. Correction: Mechanism of Snhg8/miR-384/Hoxa13/FAM3A axis regulating neuronal apoptosis in ischemic mice model. Cell Death Dis. 2019;10(9):632.
[39] Morgan EA, Nguyen SB, Scott V, et al. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130(14):3095-3109.
[40] Dong Z, Cui H. Epigenetic modulation of metabolism in glioblastoma. Semin Cancer Biol. 2019;57:45-51.
[41] Miotto B, Struhl K. HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin. Mol Cell. 2010;37(1):57-66.
[42] Feng Y, Vlassis A, Roques C, et al. BRPF3-HBO1 regulates replication origin activation and histone H3K14 acetylation. EMBO J. 2016;35(2):176-192.
[43] Sun G, Shen JF, Wei XF, et al. Circular RNA Foxo3 Relieves Myocardial Ischemia/Reperfusion Injury by Suppressing Autophagy via Inhibiting HMGB1 by Repressing KAT7 in Myocardial Infarction. J Inflamm Res. 2021;14:6397-6407.
[44] Zong H, Li Z, Liu L, et al. Cyclin-dependent kinase 11(p58) interacts with HBO1 and enhances its histone acetyltransferase activity. FEBS Lett. 2005;579(17):3579-3588.
[45] Jie M, Wu Y, Gao M, et al. CircMRPS35 suppresses gastric cancer progression via recruiting KAT7 to govern histone modification. Mol Cancer. 2020;19(1):56.
[46] Li JK, Chen C, Liu JY, et al. Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling. Mol Cancer. 2017;16(1):111.
[47] Qu D, Sun WW, Li L, et al. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019;47(6):3013-3027.
[48] Yan S, Jiang C, Li H, et al. FAM3A protects chondrocytes against interleukin-1β-induced apoptosis through regulating PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2019;516(1):209-214.
[49] Song Q, Gou WL, Zhang R. FAM3A attenuates ER stress-induced mitochondrial dysfunction and apoptosis via CHOP-Wnt pathway. Neurochem Int. 2016;94:82-89.
[50] Song Q, Gou WL, Zhang R. FAM3A Protects HT22 Cells Against Hydrogen Peroxide-Induced Oxidative Stress Through Activation of PI3K/Akt but not MEK/ERK Pathway. Cell Physiol Biochem. 2015;37(4):1431-1441.
[51] Xu W, Liang M, Zhang Y, et al. Endothelial FAM3A positively regulates post-ischaemic angiogenesis. EBioMedicine. 2019;43:32-42.
[52] Song Q, Gao Q, Chen T, et al. FAM3A Ameliorates Brain Impairment Induced by Hypoxia-Ischemia in Neonatal Rat. Cell Mol Neurobiol. 2021;doi: 10.1007/s10571-021-01172-6.
[53] Wu DC, Wang SSW, Liu CJ, et al. Reprogramming Antagonizes the Oncogenicity of HOXA13-Long Noncoding RNA HOTTIP Axis in Gastric Cancer Cells. Stem Cells. 2017;35(10):2115-2128.
[54] Shou S, Carlson HL, Perez WD, et al. HOXA13 regulates Aldh1a2 expression in the autopod to facilitate interdigital programmed cell death. Dev Dyn. 2013;242(6):687-698.
 #br#
#br#
张芳芳,女,硕士,辽宁省人民医院康复医学科技师,主要从事神经系统疾病的基础研究,发表并参与SCI论文4篇,其中一作影响因子达11.452。
赵琳,女,辽宁省人民医院康复医学科门诊护士长,长期从事神经系统疾病的康复及研究,曾发表核心期刊论文数篇。
通讯作者:张亮,男,硕士,辽宁省人民医院康复医学科主任,主要从事神经系统疾病的康复及研究,曾发表核心期刊论文数篇。
研究获得辽宁省自然科学基金资助(2021-MS-061)。
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||