NRR:中山大学胡昔权团队报道高频重复经颅磁刺激促进缺血性脑卒中后神经干细胞增殖的新机制
撰文:罗婧,奉源,洪忠秋,尹明宇,郑海清,张丽颖,胡昔权
脑卒中是中国成年人致死、致残的首位病因,已被列为中国重大疾病的防治对象。其中,以脑梗死为代表的缺血性脑卒中约占全部卒中的70-85%。脑梗死后神经细胞坏死和凋亡导致组织损伤,造成神经功能严重缺损,神经再生是组织修复的基础。成年哺乳动物中受损的神经元以前被认为是不可再生的,它们只能被神经胶质细胞取代,并随着年龄的增长而退化和逐渐减少。后来内源性神经干细胞被发现,打破了认为神经元不能再生的传统观点。但脑梗死后的神经再生数量太少,不足以修复损伤的神经功能。重复经颅磁刺激是脑卒中有效的康复治疗方法之一,近年来有文献报道,重复经颅磁刺激可激活内源性神经干细胞[1],并促进脑梗死后的神经再生,修复神经功能,但具体机制不明。
来自中国中山大学胡昔权教授团队在《中国神经再生研究(英文版)》(Neural Regeneration Research)上发表了题为“High-frequency repetitive transcranial magnetic stimulation promotes neural stem cell proliferation after ischemic stroke”的研究,发现重复经颅磁刺激可通过钙离子内流调控的磷酸化AKT/GSK3β/β-catenin信号通路来发挥促进内源性神经干细胞增殖的作用。该研究得到了重复经颅磁刺激促进缺血性脑卒中后神经功能恢复内在机制的开拓性成果,其结果可为重复经颅磁刺激临床治疗提供新的科学理论依据。
胡昔权等首先在体外和体内实验中同时验证了重复经颅磁刺激可促进缺血缺氧神经干细胞的增殖。既往有研究表明,其内在机制可能与诱导脑源性神经营养因子释放和促进Akt磷酸化[2]。但目前尚不清楚重复经颅磁刺激在促进Akt磷酸化后如何进一步促进神经干细胞的增殖。多项研究表明,经典的Wnt信号通路可以调节神经干细胞的增殖[3, 4]。转录组测序发现,重复经颅磁刺激对经典Wnt信号通路有显著的影响(图1)。进一步实验表明,重复经颅磁刺激可通过促进Akt磷酸化,上调调节细胞周期的CDK2、P21等的表达,激活糖原合成酶激酶3β/β-catenin通路,从而促进糖原合成酶激酶的增殖(图2)。最后对重复经颅磁刺激如何促进ATK磷酸化也做了进一步验证,发现重复经颅磁刺激可通过激活P2通道/CaM通路促进神经干细胞中Ca2+的流入,从而促进Akt的磷酸化并激活糖原合成酶激酶3β/β-catenin通路(图3)。
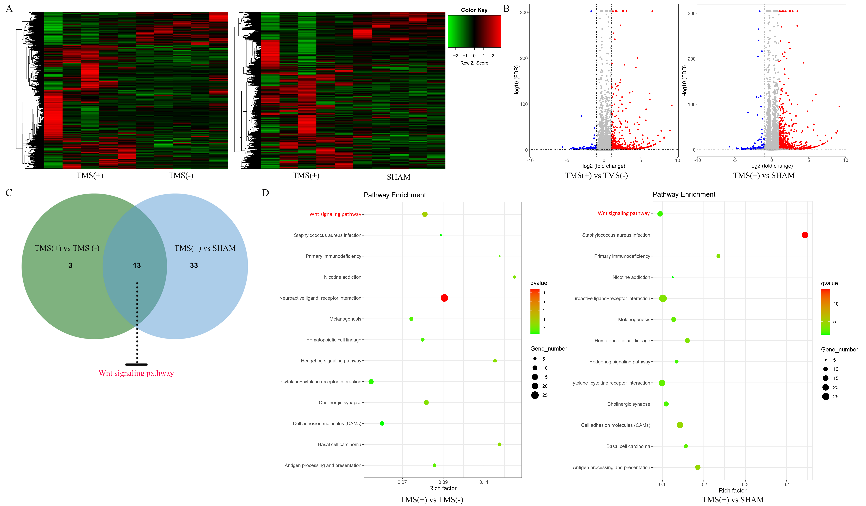
图1转录组测序结果显示重复经颅磁刺激对经典Wnt信号通路有显著的影响(图源:Luo et al., Neural Regen Res, 2024)
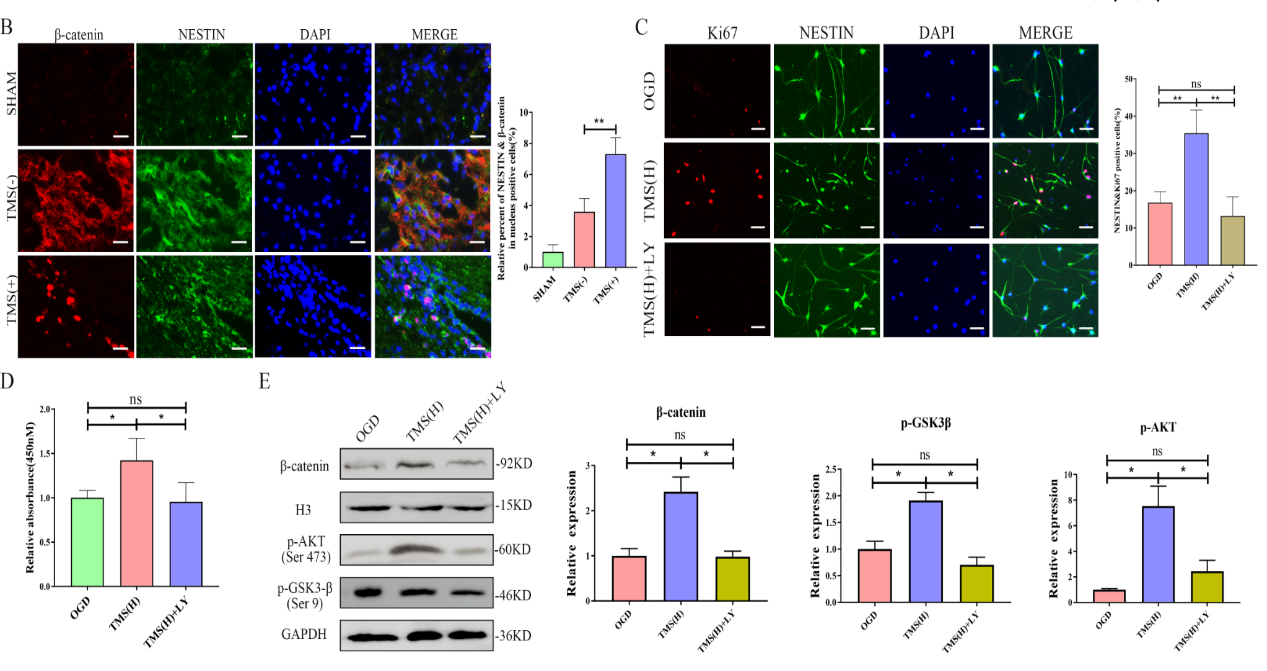
图2重复经颅磁刺激可促进Akt磷酸化激活糖原合成酶激酶3β/β-catenin通路促进神经干细胞增殖(图源:Luo et al., Neural Regen Res, 2024)
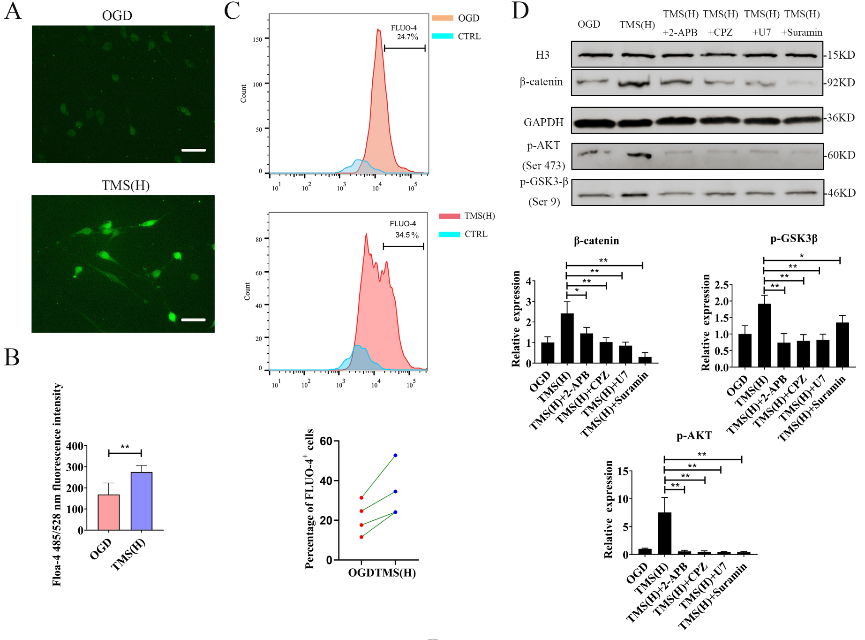
图3重复经颅磁刺激可通过激活P2通道/CaM通路促进NSC中Ca2+流入,促进Akt磷酸化(图源:Luo et al., Neural Regen Res, 2024)
该研究证明高频重复经颅磁刺激可激活Ca2+内流介导的磷酸化AKT/糖原合成酶激酶3β/β-catenin信号通路,促进神经干细胞增殖,改善缺血性脑卒中大鼠的神经功能。实验发现,重复经颅磁刺激调控神经干细胞增殖的另一种可能机制,不仅为促进神经新生以治疗脑卒中找到了一种有效且潜在无创性的方法,而且也为神经干细胞的扩增提供了一个有效的新手段,还可为重复经颅磁刺激的临床介入方向发展提供新的靶点和理论基础。由于缺血性脑卒中后的修复机制过于复杂,仅通过激活内源性神经干细胞很难达到理想的效果。在未来的研究中可将其他方法联合使用,如支持性生物材料的使用、神经康复策略的组合、药物治疗、细胞移植等。因此,在应对脑损伤的临床治疗中,需要更多的研究去发现其作用机制,优化现有的重复经颅磁刺激治疗参数,以获得更好的治疗效果。
原文链接:https://doi.org/10.4103/1673-5374.389303
参考文献
[1] Ueyama E, Ukai S, Ogawa A, et al. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci. 2011;65(1):77-81.
[2] Luo J, Zheng H, Zhang L, et al. High-frequency repetitive transcranial magnetic stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci. 2017;18(2):455.
[3] Penner R, Fasolato C, Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993;3(3):368-374.
[4] Li B, He X, Dong Z, et al. Ionomycin ameliorates hypophosphatasia via rescuing alkaline phosphatase deficiency-mediated L-type Ca(2+) channel internalization in mesenchymal stem cells. Bone Res. 2020;8:19.
第一作者:罗婧

通讯作者:胡昔权教授



