NRR:广州医科大学刘军/廖旺团队L-苏氨酸镁系列研究再获新进展:调节肠道菌群治疗阿尔茨海默病
既往研究报道,肠道菌群-肠-脑轴的紊乱可能导致阿尔茨海默病的发生。中国广州医科大学刘军和廖旺团队前期研究已发现提高镁离子浓度可促进神经干细胞定向分化[1],可减轻阿尔茨海默病小鼠的氧化应激损伤[2]。近日,该团队通过16S扩增子测序和非靶向代谢组学检测野生型、APP/PS1双转基因阿尔茨海默病小鼠模型以及L-苏氨酸镁治疗的APP/PS1双转基因小鼠的肠道菌群和血清代谢物变化,结合炎症因子和氧化应激指标的变化,阐明了L-苏氨酸镁调节肠道菌群改善阿尔茨海默病的总体机制。该发现为L-苏氨酸镁在阿尔茨海默病治疗中的临床应用提供了潜在的机制。
阿尔茨海默病是一种可由多种因素触发,且涉及多种因素机制的复杂疾病。肠道微生物对健康系统有重要影响,而肠道菌群的失调可能通过肠道菌群-肠-脑轴对中枢神经系统疾病的发病机制产生影响,具体是使病原菌进入体内并导致有毒代谢物积累。另外,神经系统疾病在发展的最初阶段常常出现胃肠道症状,许多包括肠道生态失调和免疫反应受损在内的肠道菌群失调症状都与神经退行性疾病有关。肠道菌群失调与神经退行性疾病的恶化和传播之间存在直接相关性。
肠道菌群会影响大脑功能并导致神经退行性疾病的发生,可能通过改变免疫系统的调节来影响免疫系统和神经系统之间的相互作用。肠道微生物群在大脑中产生具有抗炎和神经保护作用的代谢物。2017年的一项研究报道了阿尔茨海默病患者和健康人群肠道微生物组成的差异,几种肠道和脑脊液中的细菌相对丰度与阿尔茨海默病相关生物标志物的浓度呈正相关[3]。
镁是人体中含量第四丰富的阳离子,仅次于钠、钙和钾。它调节各种离子通道的电子流,并催化体内的许多生物和化学反应。镁可以通过抑制Na+-K+-ATP酶和镁-ATP酶来调节儿茶酚胺、阿片受体和神经DNA受体等中枢神经递质,从而影响中枢神经系统功能,并通过激素调节神经功能。在刘军和廖旺等既往研究已发现,膳食补充剂L-苏氨酸镁对APP/PS1双转基因阿尔茨海默病小鼠中有神经保护作用。
刘军和廖旺等使用L-苏氨酸镁治疗APP/PS1双转基因的阿尔茨海默病小鼠1个月,随后将L-苏氨酸镁治疗后的APP/PS1小鼠以及未经过治疗的APP/PS1小鼠置于莫里斯水迷宫中观察其运动轨迹。然后使用16S rRNA测序以及非靶向代谢组学评价L-苏氨酸镁治疗APP/PS1小鼠的肠道菌群及血清代谢物差异情况。继而以ELISA法评价L-苏氨酸镁治疗APP/PS1小鼠的炎症因子和氧化应激指标,苏木精-伊红染色评价L-苏氨酸镁对APP/PS1小鼠结肠的损害程度,蛋白印迹评价L-苏氨酸镁对APP/PS1小鼠结肠紧密连接蛋白的表达情况。结果表明,经过L-苏氨酸镁治疗可改善APP/PS1小鼠的认知能力,减轻氧化应激和炎症因子(图1)。L-苏氨酸镁还可调节APP/PS1小鼠的肠道微生物群,减少异杆菌,增加双歧杆菌和Turicibacter。此外,L-苏氨酸镁调节血清中的差异代谢产物在与神经退行性疾病相关的各种途径中富集(图2-4)。且苏木精-伊红染色对结肠进行染色以及免疫印迹法检测结肠紧密连接蛋白,发现L-苏氨酸镁会修复APP/PS1小鼠的肠道屏障功能损伤(图5)。所以,L-苏氨酸镁可能通过调节肠道菌群-肠-脑轴来减轻小鼠的阿尔茨海默病。

图1 L-苏氨酸镁改善APP/PS1小鼠的认知能力,且保护APP/PS1小鼠的结肠免受氧化应激和炎症损伤(图源:Liao et al., Neural Regen Res, 2024)
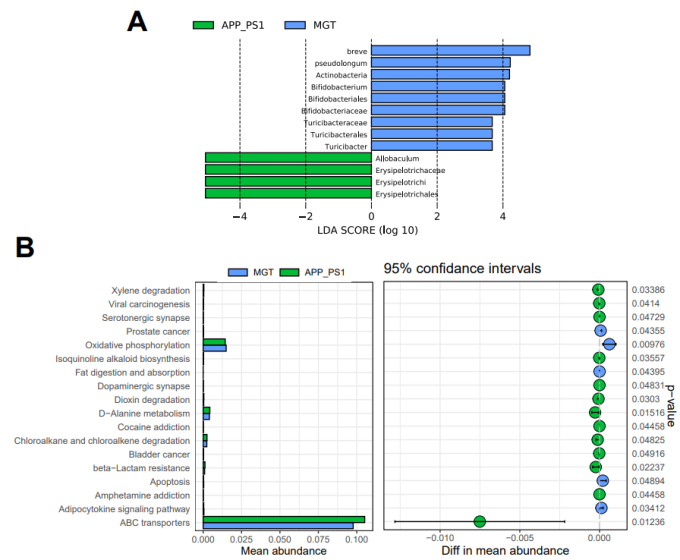
图2 L-苏氨酸镁处理的APP/PS1小鼠肠道微生物群和相关功能途径丰度的差异(图源:Liao et al., Neural Regen Res, 2024)

图3 L-苏氨酸镁治疗的APP/PS1小鼠中差异代谢产物的功能分析(图源:Liao et al., Neural Regen Res, 2024)
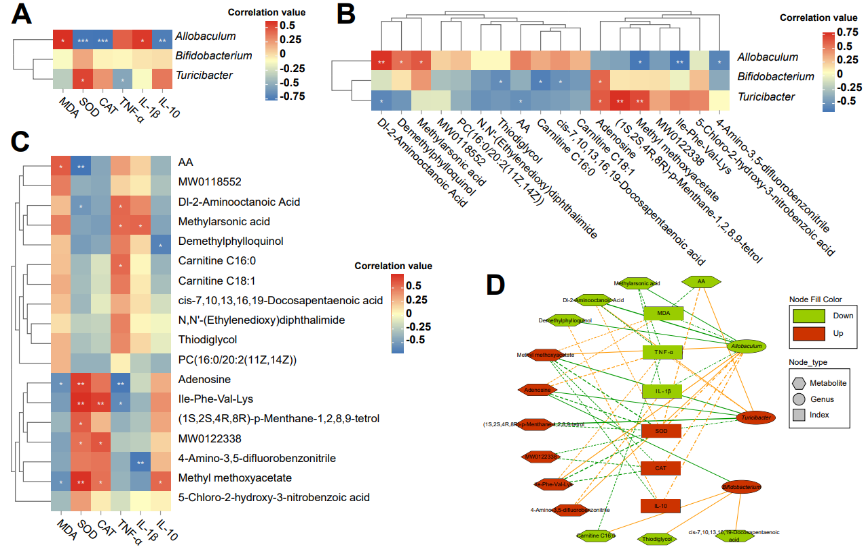
图4 L-苏氨酸镁调节APP/PS1小鼠微生物群与代谢产物的关系(图源:Liao et al., Neural Regen Res, 2024)

图5 L-苏氨酸镁修复APP/PS1小鼠的肠道屏障功能损伤(图源:Liao et al., Neural Regen Res, 2024)
此次研究结果证实L-苏氨酸镁已被证明对神经退行性疾病具有新的治疗作用。L-苏氨酸镁治疗可显著保护APP/PS1小鼠结肠免受氧化应激和炎症损伤。L-苏氨酸镁主要通过调节肠道微生物群发挥了作用,不仅能减少炎症,还能恢复紧密连接蛋白的水平,并对肠-脑轴起保护作用。未来,肠道微生物群可能成为调节神经退行性疾病的一个重要靶点。而诸如益生菌、益生元和L-苏氨酸镁之类的调节肠道微生物群的制剂可能为阿尔茨海默病治疗提供新的策略。
原文链接:https://doi.org/10.4103/1673-5374.391310
参考文献
[1] Kaur G, Behl T, Bungau S, et al. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson's Disease. Curr Neuropharmacol. 2021;19(2):233-247.
[2] Xiong Y, Ruan YT, Zhao J, et al. Magnesium-L-threonate exhibited a neuroprotective effect against oxidative stress damage in HT22 cells and Alzheimer's disease mouse model. World J Psychiatry. 2022;12(3):410-424.
3] Mothapo KM, Ten Oever J, Koopmans P, et al. Soluble TLR2 and 4 concentrations in cerebrospinal fluid in HIV/SIV-related neuropathological conditions. J Neurovirol. 2017;23(2):250-259.

通讯作者:刘军,教授、主任医师、博士生导师、留美博士后,中国十大杰出青年神经科医师,广东省杰出青年医学人才。现任广州医科大学附属第二医院神经内科主任、广州医科大学神经科学研究所副所长、国家核心高级认知障碍诊疗中心(广医二院)负责人。
长期从事神经内科的医、教、研工作,尤其是在痴呆与认知功能障碍的诊疗方面具有极深的临床造诣。牵头组建广医二院认知中心,成功入选国家核心高级认知障碍诊疗中心(华南唯一)。主持国家自然科学基金面上项目6项,省部级课题10余项,广州市重大专项2项,参与国家十三五重点研发计划“阿尔茨海默病早期诊断”1项;在Nanotoday,Biomaterials, JBC国内外重要刊物上发表SCI论文70余篇。建立了华南阿尔茨海默病多中心研究数据库,获国家计算机软件著作版权,2012年获广东省科技进步三等奖,实现了本省在该研究领域成果零的突破。2022年“氧化应激对阿尔茨海默病病理发生的影响及其干预治疗”获广东医学科技奖二等奖。

通讯作者:廖旺,广州医科大学附属第二医院神经内科主治医师、讲师,国家核心高级认知障碍诊疗中心(广医二院)秘书。中山大学、哈佛大学联合培养博士,入选“广州市青年科技人才托举工程”。
研究兴趣为阿尔茨海默病的临床与发病机制,在Advanced Functional Materials、Coordination Chemistry Reviews、Journal of Nanobiotechnology等期刊发表 SCI论文20余篇。主持科研项目13项。申请发明专利3项,建立了《华南阿尔茨海默病多中心临床研究数据库》,获国家计算机著作版权,主编专著《拒绝阿尔茨海默病》(获广州科普创新奖二等奖),参编专著2部。2019年获“全国临床医学博士后科研创新奖”,2022年获广东医学科技奖二等奖,2023年入选广州市优秀科技志愿者。
刘军/廖旺课题组长期招收博士后
单位简介
广州医科大学附属第二医院神经内科创建于1982年,拥有的神经科学研究所为省部共建教育部重点实验室和广东省神经科学疾病研究重点实验室,是我国目前唯一的神经致病基因与离子通道病方向的重点实验室。广州医科大学附属第二医院认知中心设置有广东省首个神经变性病与认知障碍专科,2022年入选华南唯一的国家核心高级认知障碍诊疗中心。拥有分子生物实验室、神经病理学实验室、细胞生物学实验室、神经免疫学实验室和动物实验室,并建立了一支结构合理的学术梯队和基础研究基地。
应聘条件
1.已经或近期将取得博士学位,学术作风严谨,对临床科学研究具有长期的热情;
2.主观能动性和团队协作精神较强;
3.具有神经病学、神经科学、基础医学相关研究背景,对认知障碍研究具有浓厚兴趣,具备独立开展科研工作能力;
4.具有较强的独立科研能力,具有较高的科研热情。
5.近期在国际重要学术期刊上发表一篇及以上一作SCI论文,较强的英文读写与交流的能力。
薪酬待遇
1.基本年薪:根据进站前论文发表情况,按Ⅰ、Ⅱ、Ⅲ档年薪分别为45万元、40万元和35万元;
2.科研绩效:根据在站期间取得的业绩成果,按Ⅰ、Ⅱ、Ⅲ类分别发放40万元、30万元、20万元的科研绩效;
3.科研经费:可申请广州市博士后科研启动费20万元;
4.科研奖励:论文、课题等业绩按广州医科大学和本院的相关规定发放。
5.课题组将为入选者的发展提供一流的科研环境和学术氛围,并支持申请国家自然科学基金、博士后基金和其他各类基金;
6.择优留院工作:博士后出站可择优留院工作,申请广州市事业编制,享受广州市安家费。
应聘材料及联系方式
请申请者将个人简历、代表性论文全文、研究工作经历发至: liaowang@gzhmu.edu.cn,邮件主题栏内请注明“应聘博士后”。






