中国神经再生研究(英文版) ›› 2026, Vol. 21 ›› Issue (4): 1574-1585.doi: 10.4103/NRR.NRR-D-23-01953
重组组织型纤溶酶原激活剂直接保护脑出血后神经元:PI3K/AKT/mTOR通路激活的作用
Recombinant tissue plasminogen activator protects neurons after intracerebral hemorrhage through activating the PI3K/AKT/mTOR pathway
Jie Jing1, 2, #, Shiling Chen1, #, Xuan Wu1 , Jingfei Yang1 , Xia Liu1 , Jiahui Wang1 , Jingyi Wang1 , Yunjie Li1 , Ping Zhang1, *, Zhouping Tang1, *
- 1 Department of Neurology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China; 2 Department of Neurology, Qilu Hospital of Shandong University, Jinan, Shandong Province, China
摘要:
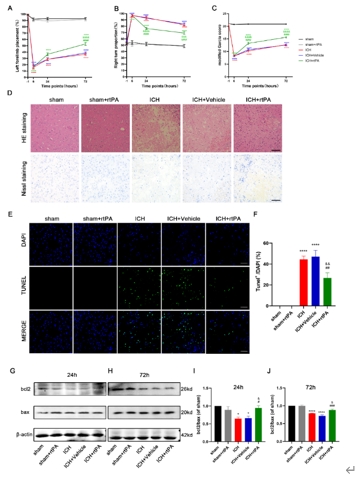
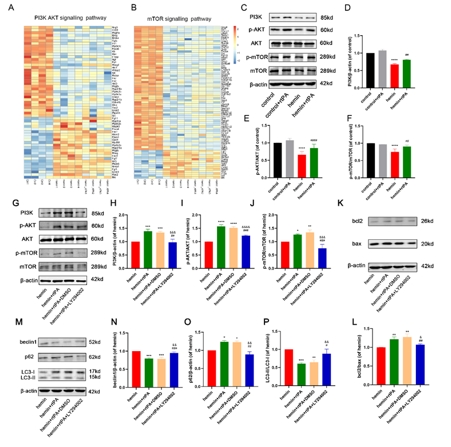
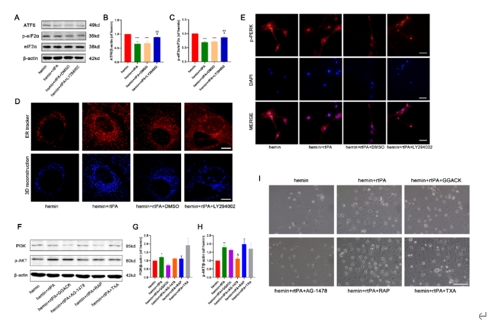
重组组织纤溶酶原激活剂是脑出血后微创手术中常用的血肿清除剂。然而,在MIS手术过程中,重组组织纤溶酶原激活剂可能直接接触脑组织,因此有必要讨论其安全性。实验首先建立了VII型胶原酶诱导的脑出血小鼠模型,发现原位重组组织型纤溶酶原激活剂给药不伴血肿抽吸即可改善脑出血小鼠的神经功能,减轻病理损伤,并降低血肿周围组织的凋亡和自噬水平。继而在血红素诱导的脑出血体外模型中,重组组织型纤溶酶原激活剂能抑制神经元的神经元凋亡、自噬和内质网应激。而后的转录组测序分析显示,重组组织型纤溶酶原激活剂还可上调神经元中PI3K/AKT/mTOR通路,而PI3K抑制剂LY294002可逆转重组组织型纤溶酶原激活剂抑制细胞凋亡、过度自噬和内质网应激的神经保护作用,且表皮生长因子受体抑制剂AG-1478可逆转重组组织型纤溶酶原激活剂对PI3K/AKT/mTOR通路的影响。上述结果表明,重组组织型纤溶酶原激活剂对脑出血后神经元有直接的神经保护作用,其可能通过激活PI3K/AKT/mTOR通路起作用。
https://orcid.org/0000-0003-0858-8884 (Ping Zhang); https://orcid.org/0000-0002-4153-8590 (Zhouping Tang)