中国神经再生研究(英文版) ›› 2026, Vol. 21 ›› Issue (4): 1652-1664.doi: 10.4103/NRR.NRR-D-24-00674
• 原著:视神经损伤修复保护与再生 • 上一篇
VDAC1寡聚化通过加剧视网膜缺血再灌注损伤中线粒体氧化应激促进泛凋亡
Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia–reperfusion injury
Hao Wan1 , Xiaoxia Ban1 , Ye He1 , Yandi Yang1 , Ximin Hu1 , Lei Shang2 , Xinxing Wan3 , Qi Zhang1, 4, 5, *, Kun Xiong1, 5, 6, *
- 1 Department of Human Anatomy and Neurobiology, School of Basic Medical Science, Central South University, Changsha, Hunan Province, China; 2 Jiangxi Research Institute of Ophthalmology and Visual Sciences, Affiliated Eye Hospital of Nanchang University, Nanchang, Jiangxi Province, China; 3 Department of Endocrinology, Third Xiangya Hospital, Central South University, Changsha, Hunan Province, China; 4 Department of Ophthalmology, Stanford University School of Medicine, Palo Alto, CA, USA; 5 Key Laboratory of Emergency and Trauma of Ministry of Education, College of Emergency and Trauma, Hainan Medical University, Haikou, Hainan Province, China; 6 Hunan Key Laboratory of Ophthalmology, Changsha, Hunan Province, China
摘要:
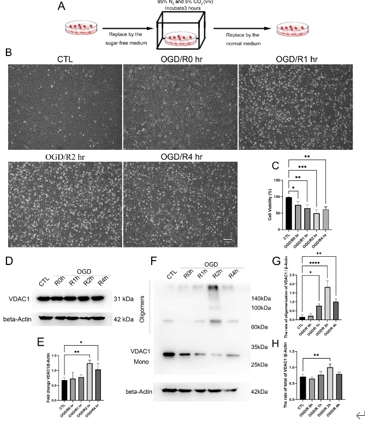
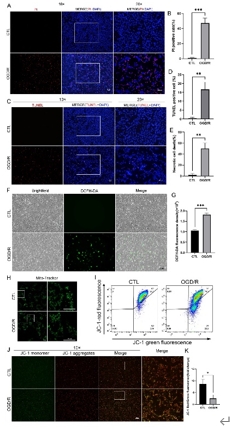
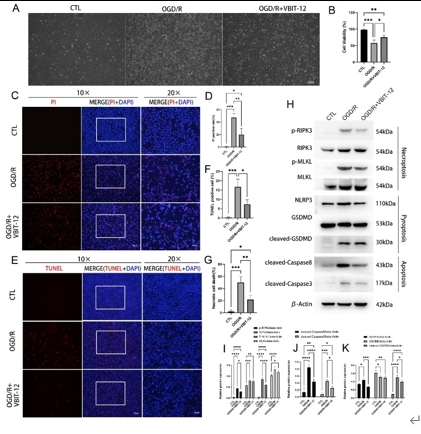
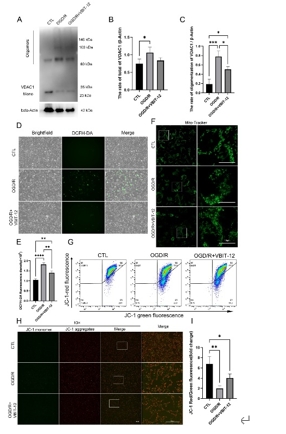
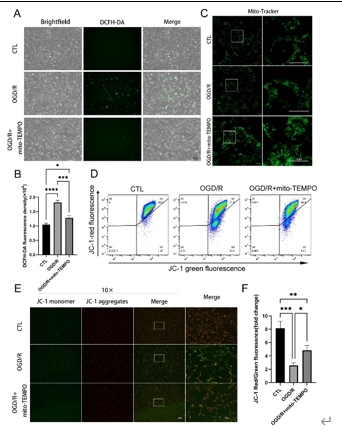
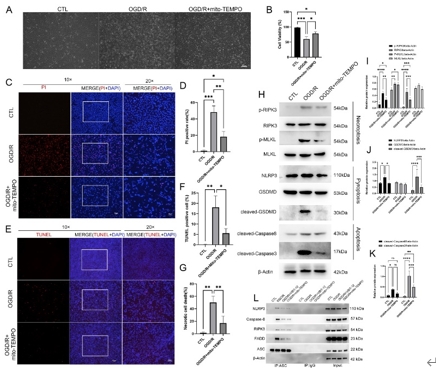
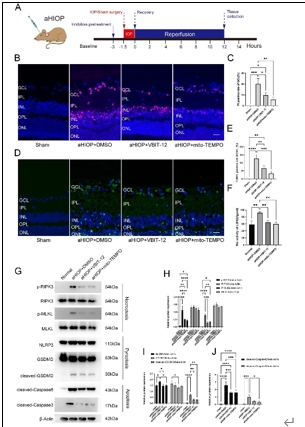
缺血再灌注损伤是视网膜变性常见的病理生理机制。泛凋亡是一种新定义的调节性细胞死亡的整体形式,其结合了焦亡、凋亡和坏死的关键特征。线粒体电压依赖性阴离子通道1(VDAC1)的寡聚化是调节视网膜缺血再灌注损伤中调节性细胞死亡的重要病理事件。然而,其在泛凋亡中的作用仍不明确。此次实验通过体内外视网膜缺血再灌注损伤模型发现,VDAC1寡聚体介导的线粒体功能障碍与视网膜缺血再灌注损伤中泛凋亡有关。抑制VDAC1寡聚化可抑制缺血再灌注损伤视网膜细胞中的线粒体功能障碍和泛凋亡。线粒体活性氧可通过促进泛凋亡小体组装,在VDAC1介导的泛凋亡中起着核心作用。此外,在缺血再灌注损伤大鼠视网膜中,也验证了抑制VDAC1寡聚化对泛凋亡的保护作用。总之,研究结果揭示了VDAC1寡聚化在调节视网膜缺血再灌注损伤泛凋亡中的重要作用,也突出了VDAC1作为有前景的治疗靶点。
https://orcid.org/0000-0002-3103-6028 (Kun Xiong); https://orcid.org/0000-0001-6300-6491 (Qi Zhang);
https://orcid.org/0000-0002-2573-6722 (Hao Wan)