NRR:青岛大学附属医院王士雷团队揭示消除边界相关巨噬细胞可减轻缺血性脑卒中的急性期损伤
撰文:于宁,王士雷
在中枢神经系统,脑常驻巨噬细胞分为实质小胶质细胞和非实质边界相关巨噬细胞(border-associated macrophage,BAM)。随着质谱流式细胞术、单细胞RNA测序等技术的发展,边界相关巨噬细胞的研究逐步深入。目前认为,边界相关巨噬细胞在调节与大脑屏障相关的神经免疫反应中发挥重要的作用,从而参与缺血性脑卒中、帕金森病、阿尔茨海默病和胶质瘤等的病理变化。有研究发现,在缺血性脑卒中中,边界相关巨噬细胞可吸收粒细胞而促进血管内皮通透性,从而加重脑损伤 [1, 2]。因此,阻断边界相关巨噬细胞引起的神经炎症是缺血性脑卒中的潜在治疗途径,但相关机制仍不清楚。
来自中国青岛大学附属医院王士雷团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Changes in border-associated
macrophages after stroke: Single-cell sequencing analysis”的研究。文章首先对基因表达综合数据库(Gene Expression Omnibus,GEO)中的数据分析发现,边界相关巨噬细胞通过介导TNF信号通路参与脑缺血再灌注损伤中的炎症反应,同时Stat3活性明显上调。之后通过构建动物模型验证发现,耗竭边界相关巨噬细胞后,脑缺血再灌注损伤明显减轻。这项研究阐明了脑卒中后边界相关巨噬细胞的转录变化,并为边界相关巨噬细胞诱导神经炎症的相关治疗策略提供新见解。
小胶质细胞、边界相关巨噬细胞和外周血单核细胞衍生巨噬细胞在脑卒中后的炎症反应中起到关键作用。目前边界相关巨噬细胞在缺血性脑卒中引起的炎症中的作用受到关注,但具体机制尚不清楚。研究旨在探讨边界相关巨噬细胞在缺血性脑卒中中的作用,以及如何通过炎症相关途径参与调节,特别是在缺血性脑卒中后边界相关巨噬细胞的转录变化和功能。研究首先整合了GEO数据库GSE174574和GSE225948中的小鼠边界相关巨噬细胞数据 [3, 4],采用单细胞RNA测序分析发现,边界相关巨噬细胞在缺血性脑卒中急性期的转录组变化主要表现为内皮细胞增殖减少和炎症增强(图1)。此外,边界相关巨噬细胞在缺血性脑卒中后通过TNF途径介导炎症反应,同时Stat3的活性在脑卒中后显著上调。然后通过构建小鼠脑缺血再灌注小鼠模型研究发现,使用含氯膦酸盐的脂质体耗竭边界相关巨噬细胞后,脑血流灌注增多,脑梗死体积和神经功能评分明显改善,同时TNF表达水平降低,但是Stat3没有显著变化(图2)。另外研究还发现,在使用TNF抑制剂后,脑血流灌注改善,梗死体积也显著减少,但神经功能评分没有显著差异(图3)。
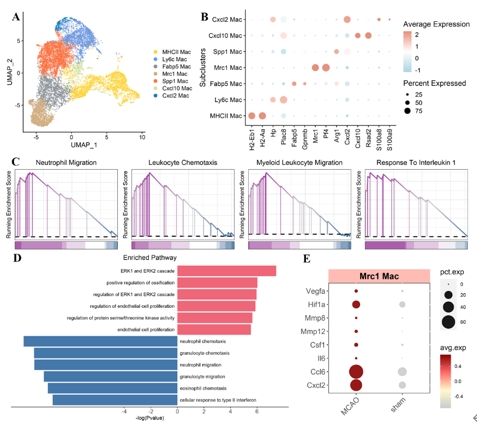
图1 边界相关巨噬细胞在缺血性脑卒中急性期的转录组变化主要表现为内皮细胞增殖减少和炎症增强(图源:Yu et al., Neural Regen Res, 2026)
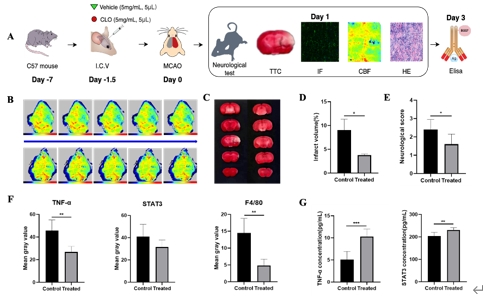
图2 在小鼠脑缺血再灌注小鼠模型中,使用含氯膦酸盐的脂质体耗竭边界相关巨噬细胞后,脑血流灌注增多,脑梗死体积和神经功能评分明显改善,同时TNF表达水平降低,但是Stat3没有显著变化(图源:Yu et al., Neural Regen Res, 2026)
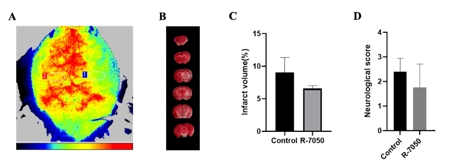
图3 使用TNF抑制剂R-7050后,脑血流灌注改善,梗死体积减少,但神经功能评分没有显著变化(图源:Yu et al., Neural Regen Res, 2026)
#br#
因此,王士雷等阐明了脑卒中后边界相关巨噬细胞的转录演化,为针对边界相关巨噬细胞诱导的神经炎症的治疗策略提供了新见解。通过耗竭边界相关巨噬细胞,可以减轻脑缺血再灌注损伤,这为缺血性脑卒中的治疗提供了新视角。当然,研究也存在一些限制和未来研究的方向。例如,单细胞RNA测序数据来自小鼠,对人类脑卒中后边界相关巨噬细胞的转录变化仍需进一步研究;此外,脑卒中后边界相关巨噬细胞在不同时间点的动态变化也需要进一步探索;同时,小胶质细胞与外周血单核细胞衍生巨噬细胞等在其中的作用也需要进一步探讨;未来的研究也需要明确边界相关巨噬细胞在脑卒中急性期和恢复期的复杂功能,以及如何平衡边界相关巨噬细胞的炎症调节和组织修复功能。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-01092
#br#
参考文献
[1] Pedragosa J, Salas-Perdomo A, Gallizioli M, et al. CNS-border associated macrophages respond to acute ischemic stroke attracting granulocytes and promoting vascular leakage. Acta Neuropathol Commun. 2018;6(1):76.
[2] Candelario-Jalil E, Dijkhuizen RM, Magnus T. Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. 2022;53(5):1473-1486.
[3] Zheng K, Lin L, Jiang W, et al. Single-cell RNA-seq reveals the transcriptional landscape in ischemic stroke. J Cereb Blood Flow Metab. 2022;42(1):56-73.
[4] Garcia-Bonilla L, Shahanoor Z, Sciortino R, et al. Analysis of brain and blood single-cell transcriptomics in acute and subacute phases after experimental stroke. Nat Immunol. 2024;25(2):357-370.


