中国神经再生研究(英文版) ›› 2026, Vol. 21 ›› Issue (6): 2295-2305.doi: 10.4103/NRR.NRR-D-24-00564
脊髓损伤中神经引导蛋白的调节作用
-
出版日期:2026-06-15发布日期:2025-09-17
Regulatory role of neuronal guidance proteins in spinal cord injury
Linyan Tang1, #, Zhi Song1, #, Jie Wang1 , Shenhua He2, *, Chao Liu2, *
- 1 Department of Intensive Care Unit, Shenzhen University General Hospital, Shenzhen, Guangdong Province, China; 2 Department of Spine Surgery, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, Guangdong Province, China
-
Online:2026-06-15Published:2025-09-17 -
Contact:Shenhua He, MS, heshenghua99@163.com; Chao Liu, PhD, liuchao413812@gmail.com. -
Supported by:This work was supported by Shenzhen University General Hospital Scientific Research Project, No. SUGH2019QD002; and Shenzhen Science and Technology Development Foundation, No. 20220810173216001 (both to ZS).
摘要:
脊髓损伤是一种严重的神经系统疾病。脊髓损伤后神经元再生和功能恢复仍然非常有限,尚无有效的方法改善脊髓损伤的预后。神经引导蛋白是在神经系统发育过程中引导轴突和树突生长的多种分子。越来越多的证据表明,神经引导蛋白对脊髓损伤有调节作用。此次综述简要介绍了神经系统形成过程中不同神经引导蛋白对神经元轴突生长的调节作用,重点介绍了神经引导蛋白对脊髓损伤后神经元再生和功能恢复的调节作用。其中神经引导蛋白包括但不限于信号蛋白(SEMA)及其受体丛蛋白、DCC和UNC5及其受体、Eph及其受体埃甫蛋白、Slit及其受体Robo、RGM及其受体neogenin、Wnt及其受体Frizzled和原粘附蛋白(Pcdh)。给予损伤部位Netrin-1可抑制成年脊髓损伤后运动轴突的再生,促进少突胶质细胞的生长。Slit2可促进大鼠受损脊髓中新突触的形成。EphA7是脊髓损伤后急性细胞凋亡的调节因子,可在脊髓损伤早期阶段起调节作用。EphrinA1可参与神经系统对损伤的反应,其表达减少可导致大鼠运动能力下降。EphA3在脊髓损伤后上调,其诱导的信号传导通路可促进了抑制轴突再生环境的形成。脊髓损伤后,ephrinB2和EphB2在星形胶质细胞和成纤维细胞中双向激活,最终促进形成星形胶质细胞-脑膜成纤维细胞瘢痕。EphB1/ephrinB1信号传导通路可通过调节神经元中的钙蛋白酶1和caspase-3介导脊髓损伤中的疼痛。脊髓损伤后白质中EphB3表达上调,后者可抑制脊髓损伤中的轴突再生。Sema3A由脊髓损伤周围瘢痕中的神经元和成纤维细胞表达,并抑制脊髓损伤后运动神经元和感觉神经的生长。Sema4D已被证明可以抑制脊髓损伤后神经元轴突的髓鞘形成和轴突再生。抑制Sema4D可显著改善脊髓损伤后的轴突再生和运动恢复。Sema7A参与脊髓损伤后胶质瘢痕的形成,可能影响脊髓损伤后血清素通道的重塑,从而影响运动协调。因此,神经引导蛋白局部或全身给药治疗脊髓损伤,具有潜在的临床应用价值。
https://orcid.org/0000-0003-3427-1573 (Linyan Tang); https://orcid.org/0009-0003-9969-913X (Chao Liu)
引用本文
. 脊髓损伤中神经引导蛋白的调节作用[J]. 中国神经再生研究(英文版), 2026, 21(6): 2295-2305.
Linyan Tang, Zhi Song, Jie Wang, Shenhua He, Chao Liu. Regulatory role of neuronal guidance proteins in spinal cord injury[J]. Neural Regeneration Research, 2026, 21(6): 2295-2305.
| [1] | . 神经系统中的电压门控钠通道:从分子生理学到治疗干预[J]. 中国神经再生研究(英文版), 2026, 21(6): 2085-2106. |
| [2] | . 低强度经颅超声神经调控技术促进神经元再生:无创治疗神经退行性疾病的新希望[J]. 中国神经再生研究(英文版), 2026, 21(6): 2275-2294. |
| [3] | . 自噬在脊髓损伤中的作用:机制、串扰和治疗策略[J]. 中国神经再生研究(英文版), 2026, 21(6): 2350-2369. |
| [4] | . 脊髓损伤后兴奋性和抑制性网状脊髓纤维可塑性差异:对功能恢复的意义[J]. 中国神经再生研究(英文版), 2026, 21(5): 2011-2020. |
| [5] | . 磁共振成像示踪超顺磁性纳米铁剂标记间充质基质细胞修复脊髓损伤的能力[J]. 中国神经再生研究(英文版), 2026, 21(5): 2031-2039. |
| [6] | . 软骨素酶 ABC 在损伤脊髓修复中的应用趋势[J]. 中国神经再生研究(英文版), 2026, 21(4): 1304-1321. |
| [7] | . 线粒体自噬:脊髓损伤病理生理和治疗的关键调节因子[J]. 中国神经再生研究(英文版), 2026, 21(4): 1396-1408. |
| [8] | . 通过靶向调控 PTEN 加强损伤脊髓中的神经干细胞整合[J]. 中国神经再生研究(英文版), 2026, 21(4): 1586-1594. |
| [9] | . 脊髓损伤与炎症介质:在“防火带”形成中的作用和神经再生潜力[J]. 中国神经再生研究(英文版), 2026, 21(3): 923-937. |
| [10] | . 脊髓损伤多靶点神经环路的重建与增强[J]. 中国神经再生研究(英文版), 2026, 21(3): 957-971. |
| [11] | . 促进NSC-34 细胞分化为稳定而持久的神经元:阿糖胞苷的作用[J]. 中国神经再生研究(英文版), 2026, 21(1): 357-364. |
| [12] | . 过表达脑源性神经营养因子的小胶质细胞可促进小鼠脊髓损伤后血管再生和功能恢复[J]. 中国神经再生研究(英文版), 2026, 21(1): 365-376. |
| [13] | . CCL2/CCR2通路成为脊髓损伤治疗靶点和调控方式的可能性[J]. 中国神经再生研究(英文版), 2025, 20(8): 2231-2244. |
| [14] | . 被动活动可增强完全性脊髓损伤患者残余运动的控制能力[J]. 中国神经再生研究(英文版), 2025, 20(8): 2337-2347. |
| [15] | . 治疗脊髓损伤的纳米粒子[J]. 中国神经再生研究(英文版), 2025, 20(6): 1665-1680. |
出版重点
《中国神经再生研究(英文版)》杂志为SCI、PubMed数据库收录的国际唯一一本专注神经再生领域研究的经同行评议的开放获取期刊,出版来自全球神经再生领域专业学者的前沿性基础研究及临床研究及转化医学、循证医学优秀的最新成果。
期刊出版来自于脑损伤与神经再生、脊髓损伤与神经再生、周围神经损伤与神经再生和神经退行性病与神经再生、神经影像与神经再生的相关研究。期刊关注神经损伤与再生过程中的轴突再生、突触生长、神经可塑性、神经修复和替代、神经移植等最新研究成果。尤其关注应用细胞治疗、基因治疗、生物因子治疗、药物治疗、手术治疗、康复治疗、物理疗法、组织工程、生物工程、生物材料、神经假体等干预性方法产生神经再生效果的相关研究。文章应清晰描述抑制神经元损伤、减轻神经元损伤的一系列变化,保护损伤神经元的过程、方法、程度与评价,突出从细胞分子水平以及分子生物学水平解释神经元损伤后以及预后再生的机制。
NRR杂志被国际重要数据库收录
科学引文索引(Science Citation Index Expanded,SCI)
美国国立医学图书馆(PubMed)
美国国立医学图书馆开放获取全文数据库(PubMed Central, PMC)
美国生物学文摘数据库(BIOSIS previews, BP)
美国《化学文摘》(Chemical Abstracts, CA)
Scopus荷兰《医学文摘库/医学文摘》(Excerpta Medica, EM)
波兰《哥伯尼索引》(Index of Copurnicus, IC)
OvidSP平台数据库
中国科学引文数据库(CSCD)
中国科技期刊数据库-统计源期刊(CSTPCD)
编委会
主编Editor-in-Chief
苏国辉院士(Kwok-fai So, Chair Professor and Head, Jessie Ho Professor in Neuroscience, Department of Anatomy, The University of Hong Kong)。
联系方式:Email: szb@nrren.org 电话:+86 138 0499 8773
编委会成员
期刊编委队伍由国际神经再生领域著名学者、中国科学院院士、香港大学苏国辉教授领导的由100多位国际神经再生优秀专家组成。共同致力于创办一本发表神经再生领域专业学术研究经同行严格评审的优秀学术期刊。
在线投稿平台
作者可以通过www.nrronline.org在线投稿。
所有的稿件都将通过该系统提交至NRR杂志电子投稿出版管理系统。
作者有不明确的问题,请访问szb@nrren.org或咨询+86 138 0499 8773。
投稿后,当论文已处于审稿或等待审稿状态时,2个月内请勿将稿件再投至他刊。
初次投稿:
投稿信:
应说明文章未一稿多投,全部作者是否对所投稿件内容知情同意,推荐2-3位小同行审稿人。
作者协议:
投稿时请注意作者协议,如同意后可继续完成投稿, 投稿成功后作者已与杂志签定了文章的相关版权。
投稿后的同行评议
期刊投稿平台应用国际最大的投稿平台Editorial Manager,投到本刊的每篇稿件都要经过3-4位小同行审稿人评审,审稿方法为国际学科小同行组成双盲审稿。所有发表在杂志的文章都将经过严格的双盲同行层层评议,审稿中注重科学性、伦理学和文章真实性的严格审查。期刊将在投稿后4周内通知作者评审意见。
根据评审意见,编辑部决定稿件返修,再审,被接受或退稿。 稿件被接受后,作者可经投稿平台以通讯作者账号随时查询稿件的出版进程。
出版时间
一般稿件被采用后3-4个月出版,优秀文章可在被采用后1-2个月内发表,有临床试验注册号的优秀临床试验文章可申请加急发表。
出版后传播
文章出版后将在EurekAlert!和EurekAlert!中文新闻平台以中英文双语形式向全世界同仁传播。EurekAlert!和EurekAlert!中文新闻平台是美国科学促进会(AAAS)主办的一项全球的科学新闻服务。美国科学促进会为世界最大的科学协会,并且是《科学》杂志的出版机构。您的文章将以最快时间推送给经严格验证的来自全球的新闻记者8000余人,包括来自国际的纽约时报、华盛顿邮报和路透社等,来自中国的中国日报、新华社、人民日报等国际主流媒体,为科学家们提供了与国际科学记者和国际学术平台直接沟通的一个快速有效的桥梁。我们将随后为您提供您新闻的网站点击情况和媒体报告情况的具体数据。
出版后的新闻传播将极大提高文章的影响力,统计同时表明发布学术新闻的文章将提高其被引率70%以上。
杂志的读者群Audience
来自全球从事神经再生、神经科学、神经解剖、神经病理、神经外科、神经内科、神经生物、神经影像、神经放射、神经康复等领域的学科专家。
特邀稿件
对特邀综述稿件的要求:
(1)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(2)全文不超过6000个单词,包括摘要,不包括参考文献,图和表格 ,出版后8-10个版面。
(3)文章写作结构:
文题:不超过 90个字母,20个单词。
摘要:非结构式, 250单词。
引言:
主体内容:
总结:
作者贡献:
利益冲突:
参考文献:采用 Journal of Neuroscience格式。
特邀述评类文章:观点、点评 、研究亮点、给编辑的信等。
特邀观点栏目文章要求:
(1)观点文章为作者对神经再生领域某一热点问题 的评论,有作者鲜明的观点和作者本人
对此科研过程的认识 和总结。
(2)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(3)全文2000- 3000单词,包括参考文献,不需要图和表格,不需要摘要,出 版后2个版面。
(4)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
特邀点评与研究亮点栏目文章 要求:
(1)点评文章为点评在本刊发表的文章,研究亮点 文章为点评国际优秀杂志近期或在线提前发表的前沿性的优秀文章。
(2)需提前向编委会提交写作大纲,通过选题后, 文章需在2个月内完成。
(3)全文2000- 3000单词,包括参考文献,不需要图和表格,不需要摘要,出 版后2个版面。
(4)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
利益冲突:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
给编辑的信栏目文章要求:
(1)给编辑的信文章为读者对本刊已发表文章的来 信反馈。
(2)全文1000- 2000单词,不包括参考文献,文章不需要摘要、图和表格,出 版后1个版面。
(3)文章写作结构:
文题:不超过 90个字母,20个单词。
主体内容:
利益冲突:
参考文献:不超过 5条,采用Journal of Neuroscience格式。
如果您需要向SCI收录期刊投稿,我们可以为您提供如下服务--
NRR:中国深圳市中医院刘超团队与深圳大学总医院汤林艳团队总结神经引导蛋白对脊髓损伤修复的调节作用
撰文:汤林艳,宋志,王杰,何升华,刘超
脊髓损伤(Spinal cord injury)是一种灾难性的神经系统疾病,通常会导致患者严重残疾或死亡,同时给医疗系统带来高昂支出和沉重负担。据估计,全球每年的脊髓损伤病例约25万到50万 [1]。脊髓损伤的临床症状包括神经源性休克、瘫痪、感觉丧失或异常,慢性疼痛、肢体痉挛,心血管、胃肠道、膀胱或性功能障碍,及严重的自主神经功能障碍 [2]。不同于周围神经的可修复性,脊髓损伤后神经元和轴突的再生能力严重受限,从而导致脊髓损伤后的各种症状及功能丧失恢复极其缓慢且微弱 [2]。脊髓损伤后的神经元和轴突的再生修复能力,受损伤脊髓内的瘢痕屏障和抑制信号的影响 [2, 3]。深入理解脊髓损伤后局部对神经元修复有调节作用的各种信号分子,能为脊髓损伤的科学研究和临床治疗探查方向,具有重要的临床和科研意义。
神经引导蛋白(neuronal guidance protein)是神经系统发育过程中对轴突和树突的生长起引导(诱导或排斥)作用的各种蛋白的总称 [4]。近年来越来越多的研究证实,部分神经引导蛋白在成熟的脊髓中仍高度表达,并在脊髓损伤后表达发生显著变化,且对脊髓损伤后神经元的修复和再生有重要的调节作用 [5-8]。
中国深圳市中医院刘超团队与深圳大学总医院汤林艳团队在《中国神经再生研究(英文)(Neural Regeneration Research)》上发表了题为“The Role of Neuronal Guidance Proteins in Spinal Cord Injury”的综述文章,通过大量的文献回顾,作者分析并系统阐明了多种神经引导蛋白对脊髓损伤后神经元再生及修复的调节(促进及抑制)作用,为脊髓损伤的科学研究和临床诊疗探查方向,具有重要的临床和科研意义。
脊髓损伤是一种灾难性的神经系统疾病,能导致神经源性休克、瘫痪、感觉丧失或异常,慢性疼痛、肢体痉挛,心血管、胃肠道、膀胱或性功能障碍及严重的自主神经功能障碍等症状 [2]。严重的脊髓损伤会导致患者严重残疾或死亡,同时给医疗系统带来高昂支出和沉重负担。据报道,2016 年美国的发病率为每百万人口54例 [11]。据估计,全球每年的脊髓损伤病例约25-50万 [1]。不同于周围神经的可修复性,脊髓损伤后神经元和轴突的再生能力严重受限,从而导致脊髓损伤后的各种症状及功能丧失恢复极其微弱且缓慢 [2]。脊髓损伤后的神经元和轴突的再生修复能力,受损伤脊髓内的瘢痕屏障和抑制信号的影响 [2, 3]。深入理解脊髓损伤后局部对神经元修复有调节作用的各种信号分子,能为脊髓损伤的科学研究和临床治疗探查方向,具有重要的临床和科研意义。
神经引导蛋白是神经系统发育过程中对轴突和树突的生长起引导(诱导或排斥)作用各种蛋白的总称 [4]。近年来越来越多的研究证实,部分神经引导蛋白在成熟的脊髓中仍然高度表达,在脊髓损伤后表达发生显著变化,且对脊髓损伤后神经元的修复和再生有重要的调节作用 [5-8]。深圳市中医院刘超团队与深圳大学总医院汤林艳团队通过回顾大量文献,系统阐明了神经引导蛋白在脊髓损伤后神经元修复过程中的作用。
1.1 神经引导蛋白的调节模式
人类神经系统是一个复杂的信息处理网络,需要处理输入感觉、分析整合信息及协调运动反应等。这些信息整合处理的解剖学基础是不同神经元树突和轴突之间建立的突触连接。在胚胎期神经系统发育过程中,轴突在复杂的细胞环境中生长延伸,与靶细胞形成突触,从而建立完整的神经回路,以实现正常的神经系统功能。由于人类中枢神经系统(由大脑和脊髓组成)平均包含861亿个神经元 [12],因此每个神经元轴突如何迁移到正确的位置是一个古老但仍未揭示的谜团。研究表明,在轴突尖端有轴突生长锥这一特殊结构,可以感知细胞外信号,并调节细胞内细胞骨架系统,来调节轴突生长锥上丝状伪足和板状伪足的延伸或收缩,进而促进其定向运动。神经引导蛋白就是这些细胞外信号蛋白的一部分。
神经引导蛋白包括多种蛋白分子,如信号素(semaphorins)及其受体丛蛋白(plexins)、netrin 及其受体(DCC和 UNC5)、Ephs 及其受体 ephrins、slit 及其受体 Robo、RGM 及其受体 neogenin、Wnt 和受体 Frizzled 以及原钙粘蛋白(Pcdhs)等 [13-16]。它们在神经系统发育过程中作为远距离或近距离的化学吸引和排斥信号发挥功能。神经引导蛋白对轴突生长的调节模式,包括自我回避信号,基于不同受体的远距离吸引或排斥信号,及近距离接触式吸引或排斥信号等(图1)。
图1 神经引导蛋白在神经系统发育形成中的调节模式
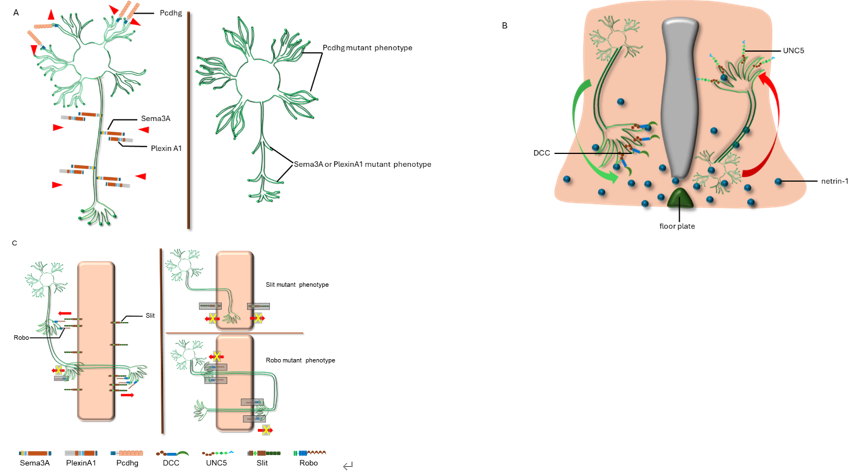
#br#
1.2 脊髓损伤的病理生理
脊髓损伤后的病理生理变化可分为原发期和继发期两个阶段 [2]。在原发期,外源性暴力导致脊髓挫伤、撕裂或横断,可直接或间接由椎骨或椎间盘碎片造成 [17]。由于血管损伤,局部组织出现出血、水肿和继发性缺血 [18]。直接暴力和缺血等因素导致脊髓损伤后1h内灰质中的神经元不可逆损伤和坏死,而白质中的神经纤维束可能存活长达72h [19]。在继发期,神经元膜损伤和缺血立即导致离子失调和神经递质积聚,进一步加剧局部组织损伤 [2]。炎症反应在脊髓损伤的继发期起着重要作用,包括中性粒细胞快速募集、驻留小胶质细胞活化、血液单核细胞浸润和瘢痕形成等 [2]。小鼠脊髓半横断后,根据募集的细胞类型,局部病变可分为中心区和周围区(图2):中心区包含活化的小胶质细胞、成纤维细胞和巨噬细胞,而周围区则充满活化的小胶质细胞、活化的星形胶质细胞、多突胶质细胞和少突胶质细胞 [20]。脊髓损伤后,星形胶质细胞被激活并表现出所谓的“反应性胶质增生”,将炎症反应限制于局部。然而,如果星形胶质细胞胶质疤痕在中枢神经系统损伤后的亚急性期没有消退,它们可能会减弱神经元再生 [2, 21]。
图2 脊髓损伤后病灶内不同类型细胞的分布
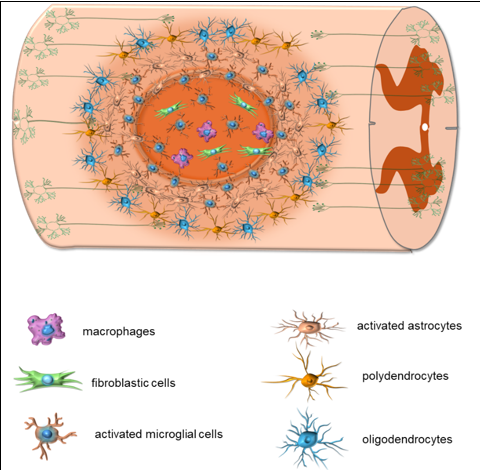
#br#
与周围神经系统相比,中枢神经系统损伤后的神经元和轴突再生能力受到极大限制 [2, 3]。这种限制是由多种机制引起的,包括瘢痕屏障和环境抑制信号 [22, 23]。脊髓损伤后,病变周围的瘢痕屏障由星形胶质细胞、小胶质细胞和成纤维细胞混合构成,它们产生致密的细胞外基质,其中含有硫酸软骨素蛋白聚糖、纤连蛋白、胶原和层粘连蛋白 [22, 23]。这些瘢痕组织在病变部位周围形成物理屏障,阻止神经元和轴突的生长和穿透,相关研究可参考综述 [22, 23]。同时,来自胶质细胞和细胞外基质的环境抑制信号在抑制神经元再生方面起着关键作用。这些抑制因子包括髓鞘抑制剂 NOGO、髓鞘相关糖蛋白(MAG)和少突胶质细胞髓鞘糖蛋白(OMGP)及其受体和辅助受体;硫酸软骨素蛋白聚糖(CSPG)、硫酸角蛋白聚糖(KSPG)、腱糖蛋白,以及多种神经引导蛋白 [2, 3]。
#br#
2.神经引导蛋白在脊髓损伤中的作用
2.1 Netrin-1在脊髓损伤中的作用
Netrin-1 从胚胎期到成年期在啮齿动物脊髓中持续表达 [24]。在成年大鼠中,Netrin-1 由各种脊髓中间神经元和运动神经元表达,包括少突胶质细胞(但不是星形胶质细胞)、背角中的中间神经元和腹角中的运动神经元。成人脊髓也表达 netrin-1 的受体,包括 DCC [25]、neogenin、UNC5h1和UNC5h2 [5]。
Löw等 [26]发现Netrin-1抑制脊髓损伤后的运动神经元再生。Netrin-1的受体UNC5仅由灰质中的红核脊髓和皮质脊髓运动神经元表达,脊髓横断后其表达降低。他们证明,体外Netrin-1可抑制神经突生长和轴突再生,因为中和Netrin-1会显著促进神经元生长。他们通过在颈髓损伤大鼠中产生移植入高表达Netrin-1的成纤维细胞,进一步证明了Netrin-1 在体内的抑制功能。与不表达Netrin的成纤维细胞移植物相比,富含Netrin-1的抑制物在损伤3个月后显著抑制了运动轴突生长并降低了轴突密度,并且这种功能依赖于运动轴突上的受体UNC5。Petit等 [27]将受损的脊髓切片与成体脊髓祖细胞共培养,也证明受损脊髓切片白质表达更多的netrin-1,抑制脊髓祖细胞的轴突生长。
然而也有研究表明,netrin-1促进脊髓损伤后少突胶质细胞上轴突和树突的生长 [28]。Lu等 [28]利用来自netrin-1 modRNA 转染的骨髓间充质干细胞的工程化外泌体(EX)研究了Netrin-1在脊髓损伤中的作用。他们发现EX-netrin 1增加了受损脊髓组织中的神经纤维密度,促进少突胶质细胞再生。少突胶质细胞表达DCC [25],这可能解释了netrin-1的促进作用。
然而,与胚胎中的高表达相比,成年大鼠脊髓中DCC和新生蛋白的表达急剧下降 [29]。相反,UNC-5表达增加 [29]。脊髓损伤后,DCC和UNC-5 的表达均急剧下降 [26, 30]。虽然UNC-5的表达在40d内恢复到正常水平 [30],但DCC的表达直到伤后7个月仍未恢复 [30]。这些信息表明,在成人脊髓中,UNC-5在Netrin-1的转导信号中起主要作用,并抑制脊髓损伤中的神经元延伸、分支和神经元再生 [29]。
与局部Netrin-1抑制脊髓损伤后神经元再生的作用相反,研究显示,血液或腹腔注射Netrin-1对急性损伤的脊髓神经元具有保护作用 [31, 32]。Gao等 [32]发现,在脊髓损伤大鼠中,腹腔注射Netrin-1明显抑制了损伤神经元的炎症反应和凋亡,对急性脊髓损伤中的神经元具有保护作用,并可能促进运动神经元的恢复。Netrin-1被证明也可以增强大鼠脊髓损伤后的神经元自噬,进而抑制神经元凋亡和丢失 [31]。
总之,损伤部位局部的Netrin-1会抑制脊髓损伤后运动轴突的再生 [26],但会促进少突胶质细胞的生长 [28]。血液或腹膜内应用 Netrin-1可通过下调局部炎症反应 [32]、增强神经元自噬 [31],进而抑制神经元凋亡来保护急性损伤的脊髓神经元 [31, 32]。
#br#
2.2 Slit和Robo在脊髓损伤中的作用
Slit是进化上保守的蛋白质家族,由Slit1-Slit3组成 [33]。它们由中枢神经系统中线胶质细胞合成,并运输至神经元轴突 [34]。Slit的受体是Robo,哺乳动物表达4种Robo亚型 (Robo1-Robo4),它们都是单次跨膜蛋白 [35]。
中线神经元轴突中的Slit通过与轴突生长锥上的Robo结合来抑制轴突穿越或重新穿越中线 [36, 37]。在成年小鼠脊髓中,神经元而非灰质中的神经胶质细胞表达Slit-1、Slit-2和Slit-3 [20]。Jacobi等 [38]采用NeuN复染进一步证明Slit表达神经元是中间神经元和运动神经元。他们还通过原位杂交表明,Slit的受体Robo-1、-2和-3由小鼠皮质脊髓侧支的投射神经元表达 [38]。脊髓背部半横断后,病变中心的细胞仍表达Slit-1和Slit-3 [20, 38],但Slit-2的表达被明显下调 [20],这些细胞通过染色显示为巨噬细胞(小胶质细胞)或成纤维细胞 [20]。
Slit-2表达的变化表明其在脊髓损伤中具有潜在作用。 Li等 [39]的研究也证实,在大鼠损伤脊髓中,Slit-2表达显著下调,同时Robo1和RhoA表达增加。用RNAi慢病毒沉默脊髓损伤大鼠中的Slit-2可导致Robo1和RhoA进一步升高。当他们鞘内注射Slit2时,脊髓损伤大鼠的运动功能恢复得更好。进一步的实验表明,应用Slit2或沉默Robo1可显著增加大鼠损伤脊髓中新的突触形成 [39]。
2.3 Eph受体及ephrin在脊髓损伤中的作用
Eph受体属于酪氨酸激酶(RTK)受体家族,其对应的配体是Ephrin [40]。根据相互亲和力和序列同源性,Eph家族蛋白分为A和B两类。EphA-ephrinA亚家族由6种EphrinA 配体(ephrinA1-ephrinA6)和10种EphA受体(EphA1-EphA10)组成,而EphB-ephrinB亚家族由3种EphrinB配体(ephrinB1-ephrinB3)和6种EphB受体(EphB1-EphB6)组成 [41, 42]。Eph家族的一个关键特征是它能够在膜结合状态下诱导双向信号传导 [40]。Eph-ephrin结合可同时触发 Eph携带细胞中的经典Eph受体酪氨酸激酶通路(“正向信号传导”)和Ephrin携带细胞中的信号通路(“反向信号传导”) [40]。这种接触介导的双向信号系统在神经系统发育中起着关键作用,包括引导皮质形成过程中的放射状胶质细胞迁移 [43],调节神经元间切向迁移 [44],促进听觉 [45]、嗅觉 [46]和躯体感觉 [47]系统中的地形映射投影,帮助中线结构的建立和胼胝体的形成 [48]。
#br#
2.3.1 EphA-ephrinA 家族在脊髓损伤中的作用
Figueroa等 [49]证明脊髓损伤后,EphA7的mRNA水平显著升高。他们利用反义寡核苷酸抑制大鼠脊髓组织中EphA7的表达,发现脊髓中凋亡细胞的密度显著降低,伤后一周后后肢运动恢复显著加快。因此,他们推定EphA7是脊髓损伤后急性细胞凋亡的调节因子,并在脊髓损伤病理生理的早期阶段发挥调节作用。
Arocho等 [50]发现,大鼠脊髓挫伤后7d,ephrinA1配体在脊髓组织中表达,到第14天显著增加并持续到第28天。双重标记显示ephrinA1配体在反应性星形胶质细胞和运动神经元中表达。行为学研究进一步表明,EphrinA1的表达降低会导致大鼠运动能力下降,这表明EphrinA1参与了神经系统对损伤的反应。
Irizarry等 [51]发现EphA3在成人健康脊髓中的基线表达较低,EphA3 mRNA水平从损伤后2d开始上升,并且这种上调持续到伤后28d。此外,EphA3诱导的信号传导促进抑制轴突再生环境的形成。这些结果表明EphA3参与了脊髓损伤的病理生理学。
#br#
2.3.2 EphB-ephrinB 家族在脊髓损伤中的作用
Song等 [52]研究发现在脊髓背根横断损伤模型中,脊髓和背根神经节中EphB1和ephrinB1的表达以时间依赖性方式显着增加。Bundesen等 [53]发现脊髓胸段横断后,ephrinB2和EphB2的蛋白水平在损伤后第一天开始上升,到第 14 天达到峰值。脊髓损伤后,ephrinB2和EphB2在星形胶质细胞和成纤维细胞内双向激活,最终导致完整的星形胶质细胞-脑膜成纤维细胞瘢痕的形成 [53]。因此,脊髓损伤后EphB2和ephrinB2表达的增加以及随后的双向信号传导促进了神经胶质瘢痕的形成,对轴突再生产生不利影响。
Yang等 [54]将ephrinB1-Fc或ephrinB2-Fc注射到实验小鼠脊髓鞘内,诱导出显著的剂量和时间依赖性热痛觉过敏和机械性异常性疼痛,随后脊髓中的caspase-3和钙蛋白酶1水平升高。此外,在小鼠的L5和L6之间施用EphB1抑制剂可预防慢性压迫性损伤引起的热痛觉过敏和机械性异常性疼痛。此外,经证实,ephrinB2-Fc 治疗后,脊髓中 caspase-3 和钙蛋白酶1 水平升高的细胞是神经元,而不是星形胶质细胞和小胶质细胞。这些结果表明,ephrinB1/EphB1 信号通过调节神经元中的钙蛋白酶1和caspase-3来介导脊髓损伤疼痛处理。
在2种大鼠脊髓损伤模型发现,脊髓损伤后7d,白质中的EphB3 mRNA水平显著升高,同时腹角灰质和中间区中EphB3 mRNA的表达也升高 [55, 56]。在大鼠模型中进行的共定位研究表明,白质星形胶质细胞和灰质运动神经元中Eph B3的表达增加。这2项研究都得出结论,EphB3的增加会抑制脊髓损伤中的轴突再生。
#br#
2.4 信号素(Semaphorin,SEMA)在脊髓损伤中的作用
信号素家族包括多种膜结合、膜表面附着或分泌型的蛋白质 [57]。根据基本结构和氨基酸序列,信号素分为8种类型 [58]。1型和2型信号素存在于无脊椎动物中,3-7型信号素存在于脊椎动物,而8型信号素存在于某些DNA病毒中 [59, 60]。信号素3属于分泌蛋白,信号素4-6是跨膜蛋白,信号素7A通过糖基磷脂酰肌醇(GPI)锚与质膜连接 [57]。信号素的主要受体包括丛蛋白(Plexin)和神经纤毛蛋白(neuropilin,NRP) [61, 62]。
#br#
2.4.1 信号素3A(Sema3A)在脊髓损伤中的作用
在成熟的神经系统中,Sema3A主要分布于大脑和脊髓的运动神经元,在神经胶质细胞中检测不到Sema3A [63]。脊髓损伤后,Sema3A与Sema3B、Sema3C、Sema3E和Sema3F一起从瘢痕中心区域的脑膜成纤维细胞中分泌 [63]。
Sema3A通过与受体PlexinA1和NRP1结合,在神经元生长中起驱动引导作用 [64]。Seong等 [65]发现Sema3A在大鼠胚胎脊髓的神经干细胞中表达,敲低Sema3A可促进大鼠受损脊髓中的细胞存活、神经干细胞的分化以及移植神经干细胞的突触连接。在大鼠脊髓损伤中,Sema3A从脊髓病变周围的神经瘢痕中分泌,脊髓下行纤维无法穿过这些Sema3A阳性的瘢痕组织 [64]。同样,也有报道称,脊髓损伤后瘢痕成纤维细胞强烈表达Sema3A,同时伴有受损背根神经节神经元分泌NRP1和plexin-A1,Sema3A阳性瘢痕形成排斥区,轴突再生受阻 [66]。
基于Sema3A对于神经元及轴突再生的明显抑制作用,研究者在脊髓损伤模型中评估了几种Sema3A抑制剂的干预效果。例如,在成年大鼠中枢神经系统创伤后,使用核心蛋白聚糖抑制神经元瘢痕组织中Sema3A的表达,证明其可促进感觉轴突生长 [67]。抑制少突胶质细胞中的Sema3A/NRP1信号传导后,受损脊髓腹角运动神经元的存活率提高,代表脊髓损伤后下肢运动功能的Basso-Beattie-Bresnahan(BBB)评分也得到改善 [68]。二聚半乳糖凝集素(Gal-1)通过糖基依赖性过程结合NRP1/PlexinA4受体复合物来抑制Sema3A,这种抑制增强了轴突再生,从而恢复运动功能 [69]。黄叶黄素(SM-216289)是一种Sema3A抑制剂,已被证实可通过增加轴突再生、髓鞘形成和血管生成,减少凋亡细胞数量来促进神经元功能恢复 [70]。表没食子儿茶素没食子酸酯的使用可减弱脊髓损伤中 Sema3A 相关的轴突抑制作用 [71]。长期以来,电针被广泛用于促进脊髓损伤的康复,电针通过促进Sema3A降解有效减少中性粒细胞的积聚,促进脊髓损伤后运动神经元的恢复 [72]。Sema3A 与神经生长因子的结合可促进脊髓失神经支配区域的靶向突触形成和轴突再生,从而促进局部功能恢复 [73]。 Sema3A 还被认为是神经生长因子反应性伤害性传入神经的抑制分子,选择性抑制成年大鼠中神经生长因子产生的神经性疼痛和神经芽生 [74]。
总之,Sema3A 由脊髓损伤周围瘢痕中的神经元和成纤维细胞表达,抑制脊髓损伤后运动神经元和感觉神经的生长。干扰或抑制 Sema3A 信号通路可促进脊髓损伤后的神经再生,促进运动和感觉功能的恢复。
#br#
2.4.2 信号素3C(Sema3C)在脊髓损伤中的作用
在胚胎中,Sema3C是大脑皮质轴突迁移的吸引信号 [75],主要以梯度浓度分布在皮质中线周围 [76-78]。它可以由胼胝体周围的谷氨酸能神经元暂时产生 [76-78]。Sema3C和Sema3B与 Sema3A竞争与NRP1结合,从而抑制Sema3A引起的生长锥塌陷,是Sema3A的拮抗剂 [79]。
在脊髓损伤中,Sema3C被证明可以调节炎症和神经元再生。在小鼠和大鼠成熟未受伤的红核脊髓神经元中,未检测到Sema3C [80]。然而,在C4水平横断左侧背外侧索引起轴突损伤后,红核脊髓神经元中 Sema3C的mRNA水平显著上调 [80]。Shen等 [81]证明小鼠钝性脊髓损伤后,Sema3C及其受体NRP-2的表达平行上升,在伤后第7天达到峰值,并在 伤后第28天时降至对照水平。他们证实了病变中心区域的巨噬细胞中Sema3C增加,并证明Sema3C在体内外促使小胶质细胞极化为促炎和促凋亡状态。Sema3C诱导炎性细胞因子释放上调,从而加剧神经元和髓鞘损伤 [81]。此外,Sema3C减弱了体外人脐静脉内皮细胞的血管生成 [81]。这种调节已被证明涉及激活 RAGE/NF-κB信号通路[81]。
#br#
2.4.3 信号素4D(Sema4D)在脊髓损伤中的作用
Sema4家族均为跨膜蛋白,在人类中有7种蛋白成员(Sema4A-4G)。在这个家族中,Sema4D 在脊髓损伤中发挥重要作用。
Sema4D,又名CD100,抑制神经系统发育过程中少突胶质细胞的分化,Sema4D缺乏会导致大脑皮质少突胶质细胞增多 [82]。Sema4D还调节γ氨基丁酸(GABA)能突触的形成,沉默 Sema4D会导致抑制性突触密度降低 [83]。Sema4D 通过结合神经系统中的plexin-B1促进生长锥的崩塌 [84]。在小鼠发育中的脊髓中,Sema4D在腹侧(运动)板中大量表达,出生后下降至无法检测到的水平 [85]。而其在脊髓白质 (少突胶质细胞) 细胞中的表达在出生后上调 [85]。
Sema4D也被证明在脊髓损伤中起着关键作用。在成年斑马鱼中,Sema4D在受伤后4 h上调,并在脊髓横断后3d内保持较高水平 [86]。增加的Sema4D源自中央管沿线的运动神经元 [86]。与 Sema4D敲除神经元相比,Sema4D阳性运动神经元在脊髓损伤的急性反应期激活了更多的小胶质细胞,并促进了脊髓损伤后的轴突再生和运动恢复 [86]。
Zhang等 [87]发现脊髓损伤大鼠Sema4D表达上调,在脊髓损伤后第一周达到峰值。Sema4D在脊髓损伤的内皮细胞和少突胶质细胞上均有表达,Sema4D的过表达促进了血管生成,但在体外抑制了神经元轴突髓鞘形成 [87, 88]。用siRNA慢病毒抑制少突胶质细胞中的Sema4D,可改善脊髓损伤大鼠的运动功能恢复 [87]。在进一步的研究中,他们证明Sema4D敲除减轻了脊髓损伤后2至3周的脊髓水肿,抑制炎性细胞因子释放,抑制血管生成因子,上调了轴突再生基因的表达,包括S100b、Erbb2、Notch1、Dcx和Drd2 [89]。
与蝾螈脊髓横断后的完全恢复能力相比,哺乳动物脊髓损伤后的神经元再生和功能恢复极其有限。Quiroz等 [90]扫描了墨西哥钝口螈和大鼠脊髓损伤后的微小RNA水平,发现两个物种之间的 miR-125b 表达存在显著差异,而Sema4D被证明是miR-125b的下游靶基因。与大鼠相比,墨西哥钝口螈脊髓损伤后的MiR-125b显著减少 [90]。抑制墨西哥钝口螈体内的 miR-125b会导致损伤部位Sema4D表达增加,从而导致脊髓损伤后再生缺陷 [90]。此外,Sema4D的过表达显著抑制了蝾螈的轴突再生,而体外星形胶质细胞中Sema4D的消耗促进了受损神经元的轴突生长 [90]。
这些研究中证明的Sema4D的显著抑制作用,促使了Sema4D在脊髓损伤临床前阶段的应用研究。Li等 [91]构建了胶原结合蛋白CBD-EphA4LBD和CBD-PlexinB1LBD来中和ephrinB3和Sema4D。他们将固定了CBD-EphA4LBD和CBD-PlexinB1LBD的胶原支架移植到脊髓损伤大鼠体内,发现轴突再生和运动恢复得到了显著改善。
#br#
2.4.4 信号素7A(Sema7A)在脊髓损伤中的作用
Sema7A 是一种糖基磷脂酰肌醇(GPI)连接的膜信号蛋白 [92, 93],以PlexinC1和β1整合素结合作为受体 [94]。越来越多的研究表明Sema7A在脊髓损伤中起重要作用。脊髓损伤后,Sema7A 表达在病变部位上调,但在邻近区域则无变化 [95]。Sema7A的增强表达在伤后14d达高峰,并持续28d [95]。神经元损伤后,Sema7A沉积在回缩球和肿胀的轴突中,然后被吞噬并转移到病变中心区的巨噬细胞溶酶体包涵体中 [95]。在损伤后14d,反应性星形胶质细胞高度表达Sema7A,并在形成的神经胶质瘢痕中持续4周\ [95]。这些结果表明,Sema7A 参与了脊髓损伤后的神经胶质瘢痕形成,并可能影响神经元再生。
血清素能神经元合成血清素(5-HT)作为神经递质,来调节运动并协调姿势和运动节奏 [96, 97]。Loy等 [98]研究表明,小鼠中Sema7A缺失导致成年鼠脊髓所有层的血清素能神经元密度显著增加。在脊髓损伤小鼠中,Sema7A对于步态和运动的正确恢复至关重要,因为缺乏Sema7A的小鼠表现出更差的运动能力 [98]。这表明Sema7A也可能影响脊髓损伤中的血清素通道重塑,进而影响运动和姿势的协调。
综上所述,脊髓损伤是一种灾难性的神经系统疾病,通常会导致严重的残疾和死亡,给医疗保健系统和个人带来沉重的医疗负担。成人脊髓损伤后神经元的再生和功能恢复受到极大限制,所涉及的机制包括瘢痕的机械性阻挡和局部抑制信号的影响。多种神经引导蛋白已被证明在调节脊髓损伤后的炎症反应、神经元凋亡以及神经元及轴突的再生中起着重要作用。例如,损伤部位的局部 Netrin-1抑制成人脊髓损伤后运动轴突的再生 [26],但会促进少突胶质细胞的生长 [28]。血液或腹膜内应用 Netrin-1可通过下调局部炎症反应 [32]、增强神经元自噬 [31],进而抑制神经元凋亡,来保护急性损伤的脊髓神经元 [31, 32]。Slit2可显著增加大鼠损伤脊髓中新的突触形成 [39]。EphA7是脊髓损伤后急性细胞凋亡的调节因子,并在脊髓损伤病理生理的早期阶段发挥调节作用 [49]。EphrinA1参与了神经系统对损伤的反应,EphrinA1的表达降低会导致大鼠运动能力下降 [50]。EphA3在脊髓损伤后表达上调,而其诱导的信号传导促进抑制轴突再生环境的形成 [51]。脊髓损伤后,ephrinB2和EphB2 在星形胶质细胞和成纤维细胞内双向激活,最终导致完整的星形胶质细胞-脑膜成纤维细胞瘢痕的形成 [53]。EphB1/ephrinB1信号通过调节神经元中的钙蛋白酶1和caspase-3来介导脊髓损伤疼痛处理。脊髓损伤后EphB3 在白质中表达增加,并抑制脊髓损伤中的轴突再生 [55, 56]。Sema3A 由脊髓损伤周围瘢痕中的神经元和成纤维细胞表达,抑制脊髓损伤后运动神经元和感觉神经的生长。干扰或抑制Sema3A信号通路可促进脊髓损伤后的神经再生,促进运动和感觉功能的恢复 [67]。Sema3C在脊髓损伤后表达增强,并能在体内和体外促使小胶质细胞极化为促炎和促凋亡状态,从而加剧神经元和髓鞘损伤 [81]。Sema4D被证明抑制脊髓损伤后神经元轴突髓鞘形成 [87, 88]和轴突再生 [90],抑制Sema4D显著改善了脊髓损伤后的轴突再生和运动恢复 [91]。Sema7A参与了脊髓损伤后的神经胶质瘢痕形成 [95],并可能影响脊髓损伤中的血清素通道重塑,进而影响运动协调 [98]。基于上述理论,局部或全身应用神经引导蛋白治疗脊髓损伤,是一个很有前景的研究领域。
#br#
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-00564
#br#
参考文献
[1] Khorasanizadeh M, Yousefifard M, Eskian M, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine. 2019;30(5):683-699.
[2] Quadri SA, Farooqui M, Ikram A, et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43(2):425-441.
[3] Zheng B, Tuszynski MH. Regulation of axonal regeneration after mammalian spinal cord injury. Nat Rev Mol Cell Biol. 2023;24(6):396-413.
[4] Mirakaj V, Rosenberger P. Immunomodulatory functions of neuronal guidance proteins. Trends Immunol. 2017;38(6):444-456.
[5] Manitt C, Colicos MA, Thompson KM, et al. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J Neurosci. 2001;21(11):3911-3922.
[6] Tanno T, Fujiwara A, Takenaka S, et al. Expression of a chemorepellent factor, Slit2, in peripheral nerve regeneration. Biosci Biotechnol Biochem. 2005;69(12):2431-2434.
[7] Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1499-1511.
[8] Parrinello S, Napoli I, Ribeiro S, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143(1):145-155.
[9] Spinal Cord Injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med. 2016;39(4):493-494.
[10] Barbiellini Amidei C, Salmaso L, Bellio S, et al. Epidemiology of traumatic spinal cord injury: a large population-based study. Spinal Cord. 2022;60(9):812-819.
[11] Ning GZ, Wu Q, Li YL, et al. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35(4):229-239.
[12] Herculano-Houzel S, Kaas JH, De Oliveira-Souza R. Corticalization of motor control in humans is a consequence of brain scaling in primate evolution. J Comp Neurol. 2016;524(3):448-455.
[13] Pasterkamp RJ, Kolodkin AL. SnapShot: Axon Guidance. Cell. 2013;153(2):494, 494e491-492.
[14] Kolodkin AL, Pasterkamp RJ. SnapShot: Axon guidance II. Cell. 2013;153(3):722.e721.
[15] Stoeckli ET. Understanding axon guidance: are we nearly there yet? Development. 2018;145(10):dev151415.
[16] Leonardo ED, Hinck L, Masu M, et al. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386(6627):833-838.
[17] Dumont RJ, Verma S, Okonkwo DO, et al. Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol. 2001;24(5):265-279.
[18] Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75(1):15-26.
[19] Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989;91(1-2):15-34.
[20] Wehrle R, Camand E, Chedotal A, et al. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur J Neurosci. 2005;22(9):2134-2144.
[21] Goldshmit Y, Galea MP, Wise G, et al. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24(45):10064-10073.
[22] Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15(3):541-553.
[23] Clifford T, Finkel Z, Rodriguez B, et al. Current advancements in spinal cord injury research-glial scar formation and neural regeneration. Cells. 2023;12(6):853.
[24] Serafini T, Kennedy TE, Galko MJ, et al. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78(3):409-424.
[25] Rajasekharan S, Baker KA, Horn KE, et al. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136(3):415-426.
[26] Löw K, Culbertson M, Bradke F, et al. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28(5):1099-1108.
[27] Petit A, Sellers DL, Liebl DJ, et al. Adult spinal cord progenitor cells are repelled by netrin-1 in the embryonic and injured adult spinal cord. Proc Natl Acad Sci U S A. 2007;104(45):17837-17842.
[28] Lu X, Xu G, Lin Z, et al. Engineered exosomes enriched in netrin-1 modRNA promote axonal growth in spinal cord injury by attenuating inflammation and pyroptosis. Biomater Res. 2023;27(1):3.
[29] Manitt C, Thompson KM, Kennedy TE. Developmental shift in expression of netrin receptors in the rat spinal cord: predominance of UNC-5 homologues in adulthood. J Neurosci Res. 2004;77(5):690-700.
[30] Manitt C, Wang D, Kennedy TE, et al. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. J Neurosci Res. 2006;84(8):1808-1820.
[31] Bai L, Mei X, Wang Y, et al. The role of netrin-1 in improving functional recovery through autophagy stimulation following spinal cord injury in rats. Front Cell Neurosci. 2017;11:350.
[32] Gao K, Niu J, Dang X. Neuroprotection of netrin-1 on neurological recovery via Wnt/β-catenin signaling pathway after spinal cord injury. Neuroreport. 2020;31(7):537-543.
[33] Blockus H, Chédotal A. Slit-Robo signaling. Development. 2016;143(17):3037-3044.
[34] Rothberg JM, Jacobs JR, Goodman CS, et al. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4(12a):2169-2187.
[35] Gonda Y, Namba T, Hanashima C. Beyond axon guidance: roles of Slit-Robo signaling in neocortical formation. Front Cell Dev Biol. 2020;8:607415.
[36] Kidd T, Brose K, Mitchell KJ, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92(2):205-215.
[37] Battye R, Stevens A, Jacobs JR. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development. 1999;126(11):2475-2481.
[38] Jacobi A, Schmalz A, Bareyre FM. Abundant expression of guidance and synaptogenic molecules in the injured spinal cord. PLoS One. 2014;9(2):e88449.
[39] Li Y, Gao Y, Xu X, et al. Slit2/Robo1 promotes synaptogenesis and functional recovery of spinal cord injury. Neuroreport. 2017;28(2):75-81.
[40] Egea J, Klein R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007;17(5):230-238.
[41] Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 2009;12(1):15-20.
[42] Aoto J, Chen L. Bidirectional ephrin/Eph signaling in synaptic functions. Brain Res. 2007;1184:72-80.
[43] He S, Li Z, Ge S, et al. Inside-out radial migration facilitates lineage-dependent neocortical microcircuit assembly. Neuron. 2015;86(5):1159-1166.
[44] Zimmer G, Rudolph J, Landmann J, et al. Bidirectional ephrinB3/EphA4 signaling mediates the segregation of medial ganglionic eminence- and preoptic area-derived interneurons in the deep and superficial migratory stream. J Neurosci. 2011;31(50):18364-18380.
[45] Miko IJ, Nakamura PA, Henkemeyer M, et al. Auditory brainstem neural activation patterns are altered in EphA4- and ephrin-B2-deficient mice. J Comp Neurol. 2007;505(6):669-681.
[46] Serizawa S, Miyamichi K, Takeuchi H, et al. A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell. 2006;127(5):1057-1069.
[47] Prakash N, Vanderhaeghen P, Cohen-Cory S, et al. Malformation of the functional organization of somatosensory cortex in adult ephrin-A5 knock-out mice revealed by in vivo functional imaging. J Neurosci. 2000;20(15):5841-5847.
[48] Twigg SR, Kan R, Babbs C, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc Natl Acad Sci U S A. 2004;101(23):8652-8657.
[49] Figueroa JD, Benton RL, Velazquez I, et al. Inhibition of EphA7 up-regulation after spinal cord injury reduces apoptosis and promotes locomotor recovery. J Neurosci Res. 2006;84(7):1438-1451.
[50] Arocho LC, Figueroa JD, Torrado AI, et al. Expression profile and role of EphrinA1 ligand after spinal cord injury. Cell Mol Neurobiol. 2011;31(7):1057-1069.
[51] Irizarry-Ramírez M, Willson CA, Cruz-Orengo L, et al. Upregulation of EphA3 receptor after spinal cord injury. J Neurotrauma. 2005;22(8):929-935.
[52] Song XJ, Cao JL, Li HC, et al. Upregulation and redistribution of ephrinB and EphB receptor in dorsal root ganglion and spinal dorsal horn neurons after peripheral nerve injury and dorsal rhizotomy. Eur J Pain. 2008;12(8):1031-1039.
[53] Bundesen LQ, Scheel TA, Bregman BS, et al. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23(21):7789-7800.
[54] Yang M, Chen W, Zhang Y, et al. EphrinB/EphB signaling contributes to spinal nociceptive processing via calpain‑1 and caspase‑3. Mol Med Rep. 2018;18(1):268-278.
[55] Miranda JD, White LA, Marcillo AE, et al. Induction of Eph B3 after spinal cord injury. Exp Neurol. 1999;156(1):218-222.
[56] Willson CA, Miranda JD, Foster RD, et al. Transection of the adult rat spinal cord upregulates EphB3 receptor and ligand expression. Cell Transplant. 2003;12(3):279-290.
[57] Alto LT, Terman JR. Semaphorins and their signaling mechanisms. Methods Mol Biol. 2017;1493:1-25.
[58] Unified nomenclature for the semaphorins/collapsins. Semaphorin Nomenclature Committee. Cell. 1999;97(5):551-552.
[59] Pasterkamp RJ, Kolodkin AL. Semaphorin junction: making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13(1):79-89.
[60] Spriggs MK. Shared resources between the neural and immune systems: semaphorins join the ranks. Curr Opin Immunol. 1999;11(4):387-391.
[61] Tamagnone L, Artigiani S, Chen H, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71-80.
[62] Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov. 2014;13(8):603-621.
[63] Niclou SP, Ehlert EM, Verhaagen J. Chemorepellent axon guidance molecules in spinal cord injury. J Neurotrauma. 2006;23(3-4):409-421.
[64] De Winter F, Oudega M, Lankhorst AJ, et al. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp Neurol. 2002;175(1):61-75.
[65] Kim SJ, Ko WK, Han GH, et al. Axon guidance gene-targeted siRNA delivery system improves neural stem cell transplantation therapy after spinal cord injury. Biomater Res. 2023;27(1):101.
[66] Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13(3):457-471.
[67] Minor KH, Bournat JC, Toscano N, et al. Decorin, erythroblastic leukaemia viral oncogene homologue B4 and signal transducer and activator of transcription 3 regulation of semaphorin 3A in central nervous system scar tissue. Brain. 2011;134(Pt 4):1140-1155.
[68] Hu R, Shi M, Xu H, et al. Integrated bioinformatics analysis identifies the effects of Sema3A/NRP1 signaling in oligodendrocytes after spinal cord injury in rats. PeerJ. 2022;10:e13856.
[69] Quintá HR, Pasquini JM, Rabinovich GA, et al. Glycan-dependent binding of galectin-1 to neuropilin-1 promotes axonal regeneration after spinal cord injury. Cell Death Differ. 2014;21(6):941-955.
[70] Tohda C, Kuboyama T. Current and future therapeutic strategies for functional repair of spinal cord injury. Pharmacol Ther. 2011;132(1):57-71.
[71] Pasterkamp RJ, Dai HN, Terman JR, et al. MICAL flavoprotein monooxygenases: expression during neural development and following spinal cord injuries in the rat. Mol Cell Neurosci. 2006;31(1):52-69.
[72] Hu R, Xu H, Jiang Y, et al. EA Improves the motor function in rats with spinal cord injury by inhibiting signal transduction of Semaphorin3A and upregulating of the peripheral nerve networks. Neural Plast. 2020;2020:8859672.
[73] Tang XQ, Heron P, Mashburn C, et al. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27(22):6068-6078.
[74] Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24(4):819-827.
[75] Bagnard D, Lohrum M, Uziel D, et al. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125(24):5043-5053.
[76] Mire E, Hocine M, Bazellières E, et al. Developmental upregulation of Ephrin-B1 silences Sema3C/Neuropilin-1 signaling during post-crossing navigation of corpus callosum axons. Curr Biol. 2018;28(11):1768-1782.e1764.
[77] Ruediger T, Zimmer G, Barchmann S, et al. Integration of opposing semaphorin guidance cues in cortical axons. Cereb Cortex. 2013;23(3):604-614.
[78] Niquille M, Garel S, Mann F, et al. Transient neuronal populations are required to guide callosal axons: a role for semaphorin 3C. PLoS Biol. 2009;7(10):e1000230.
[79] Takahashi T, Nakamura F, Jin Z, et al. Semaphorins A and E act as antagonists of neuropilin-1 and agonists of neuropilin-2 receptors. Nat Neurosci. 1998;1(6):487-493.
[80] Oschipok LW, Teh J, Mcphail LT, et al. Expression of Semaphorin3C in axotomized rodent facial and rubrospinal neurons. Neurosci Lett. 2008;434(1):113-118.
[81] Shen J, Gong L, Sun Y, et al. Semaphorin3C identified as mediator of neuroinflammation and microglia polarization after spinal cord injury. iScience. 2024;27(5):109649.
[82] Yamaguchi W, Tamai R, Kageura M, et al. Sema4D as an inhibitory regulator in oligodendrocyte development. Mol Cell Neurosci. 2012;49(3):290-299.
[83] Raissi AJ, Staudenmaier EK, David S, et al. Sema4D localizes to synapses and regulates GABAergic synapse development as a membrane-bound molecule in the mammalian hippocampus. Mol Cell Neurosci. 2013;57:23-32.
[84] Ito Y, Oinuma I, Katoh H, et al. Sema4D/plexin-B1 activates GSK-3beta through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep. 2006;7(7):704-709.
[85] Worzfeld T, Püschel AW, Offermanns S, et al. Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development. Eur J Neurosci. 2004;19(10):2622-2632.
[86] Peng SX, Yao L, Cui C, et al. Semaphorin4D promotes axon regrowth and swimming ability during recovery following zebrafish spinal cord injury. Neuroscience. 2017;351:36-46.
[87] Zhang HL, Wang J, Tang L. Sema4D knockdown in oligodendrocytes promotes functional recovery after spinal cord injury. Cell Biochem Biophys. 2014;68(3):489-496.
[88] Moreau-Fauvarque C, Kumanogoh A, Camand E, et al. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J Neurosci. 2003;23(27):9229-9239.
[89] Zhang HL, Jiang ZS, Wang FW. Analysis of gene expression profiles associated with functional recovery after spinal cord injury caused by sema4D knockdown in oligodendrocytes. Cell Biochem Biophys. 2014;69(3):655-661.
[90] Diaz Quiroz JF, Tsai E, Coyle M, et al. Precise control of miR-125b levels is required to create a regeneration-permissive environment after spinal cord injury: a cross-species comparison between salamander and rat. Dis Model Mech. 2014;7(6):601-611.
[91] Li X, Han J, Zhao Y, et al. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl Mater Interfaces. 2015;7(25):13960-13971.
[92] Pasterkamp RJ, Peschon JJ, Spriggs MK, et al. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424(6947):398-405.
[93] Bobolis KA, Moulds JJ, Telen MJ. Isolation of the JMH antigen on a novel phosphatidylinositol-linked human membrane protein. Blood. 1992;79(6):1574-1581.
[94] Pasterkamp RJ, Kolk SM, Hellemons AJ, et al. Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol. 2007;7:98.
[95] Kopp MA, Brommer B, Gatzemeier N, et al. Spinal cord injury induces differential expression of the profibrotic semaphorin 7A in the developing and mature glial scar. Glia. 2010;58(14):1748-1756.
[96] Ballion B, Branchereau P, Chapron J, et al. Ontogeny of descending serotonergic innervation and evidence for intraspinal 5-HT neurons in the mouse spinal cord. Brain Res Dev Brain Res. 2002;137(1):81-88.
[97] Takakusaki K, Saitoh K, Harada H, et al. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res. 2004;50(2):137-151.
[98] Loy K, Fourneau J, Meng N, et al. Semaphorin 7A restricts serotonergic innervation and ensures recovery after spinal cord injury. Cell Mol Life Sci. 2021;78(6):2911-2927.
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||