NRR:中国首都医科大学宣武医院江荣才团队提出靶向鼠双微体同源基因2和谷胱甘肽过氧化物酶4治疗脑出血有前景
脑出血多指的是原发性非外伤性脑实质内出血,具有致残、致死率高的特点[1-2]。脑出血的病理机制主要包括血肿的占位效应、血肿分解产物释放出的血管活性物质等所致的原发性及继发性损伤等[3]。虽然临床治疗措施不断进步,但是脑出血后的死亡率在过去的几十年没有明显改善[4],原因之一可能是脑出血的继发性损伤机制复杂,目前尚没有重要的治疗靶点被发现,导致其总体治愈率距离预期仍有非常大的差距。尽管脑出血是人类发病和死亡的主要原因,但其广泛神经损伤的机制仍不完全清楚。越来越多的证据表明,小胶质细胞作为中枢神经系统中的常驻巨噬细胞,是对各种急性脑损伤作出反应的第一个非神经元细胞[5,6]。研究脑出血后小胶质细胞对损伤的反应机制及其细胞死亡模式是一个迫切的研究领域,可以使许多患者受益。
近期,天津医科大学总医院&首都医科大学宣武医院江荣才团队在《中国神经再生研究(英文)》(Neural
Regeneration Research)上发表的研究证明抑制鼠双微体同源基因2可以减轻脑出血诱导的神经功能障碍和氧糖剥夺联合血红素诱导的脑小胶质细胞损伤,突出了其在治疗脑出血诱导的继发性脑损伤方面的潜在应用。此外,鼠双微体同源基因2与谷胱甘肽过氧化物酶4相互作用,鼠双微体同源基因2通过促进其泛素化而下调谷胱甘肽过氧化物酶4蛋白水平,从而导致氧糖剥夺结合血红素氧糖剥夺结合血红素诱导的小胶质细胞功能障碍。这些发现表明:靶向鼠双微体同源基因2和谷胱甘肽过氧化物酶4是一种很有前景的脑出血治疗策略。余云湖和刘涛为论文共同第一作者,江荣才教授和遵义医科大学附属医院曹芳教授为论文通讯作者。
在脑出血发生后,鼠双微体同源基因2通过诱导谷胱甘肽过氧化物酶4的泛素化和降解,以调节BV2小胶质细胞的细胞死亡和炎症反应;再进一步研究鼠双微体同源基因2在脑出血中的分子调控机制,揭示鼠双微体同源基因2在介导小胶质细胞极化过程中的分子网络。该研究结果不仅能够加深对于脑出血分子机制的理解,而且为提高和改善现有的脑出血靶向治疗措施提供了新的潜在靶点及数据支撑。有助于认识细胞极化在脑出血疾病发生发展和转归中的作用,为临床防治提供新思路。首先,在氧糖剥夺结合血红素处理不同时间的BV2小胶质细胞中检测鼠双微体同源基因2的表达。如图1A和1B所示,随着氧糖剥夺结合血红素处理时间的延长,鼠双微体同源基因2表达显著增加。然后用鼠双微体同源基因2干扰RNA及其阴性对照shNC转导BV2小胶质细胞。定量RT-PCR和Western blotting证实在指定的细胞中成功干扰MDM2表达(图1C)。氧糖剥夺结合血红素处理后,细胞活力显著降低,LDH活性升高。相比之下,鼠双微体同源基因2干扰RNA显著提高细胞活力,降低LDH活性(图1E和1F)。如图1G-1K所示,氧糖剥夺结合血红素降低GSH含量,增加MDA含量,增加亚铁离子含量和脂质ROS水平,抑制谷胱甘肽过氧化物酶4表达。此外,氧糖剥夺结合血红素增加了M1极化标记物iNOS、IL-1β和TNF-α的mRNA表达和分泌,抑制了M2极化标记物Arg-1、IL-10和TGF-β的mRNA表达和分泌,表明其在调节炎症反应中的作用(图1L-1N)。然而,这些作用在鼠双微体同源基因2干扰RNA转导后被逆转(图1G-1N)。这些结果表明,鼠双微体同源基因2干扰可抑制氧糖剥夺结合血红素诱导的BV2小胶质细胞的铁死亡和炎症反应。
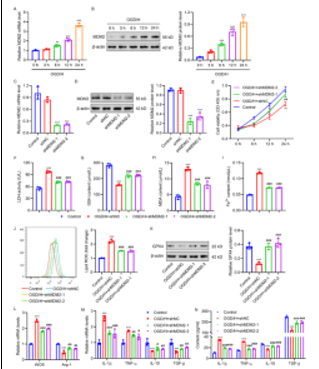
图1 干扰鼠双微体同源基因2抑制氧糖剥夺结合血红素诱导的BV2小胶质细胞铁死亡和M1/M2极化
免疫共沉淀、泛素化和Western blotting实验发现,鼠双微体同源基因2通过结合谷胱甘肽过氧化物酶4诱导其泛素化降解减弱谷胱甘肽过氧化物酶4蛋白稳定性(图2A-2G)。同时谷胱甘肽过氧化物酶4基因干扰能够抑制鼠双微体同源基因2基因干扰所产生的作用,例如促进BV2小胶质细胞铁死亡和M1/M2极化(图3A-3L)。这些结果表明,鼠双微体同源基因2诱导的谷胱甘肽过氧化物酶4泛素化和降解影响氧糖剥夺结合血红素诱导的BV2小胶质细胞的铁死亡和炎症反应。
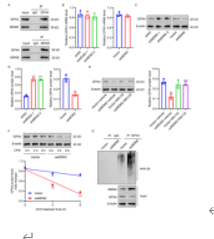
图2鼠双微体同源基因2诱导谷胱甘肽过氧化物酶4泛素化降解
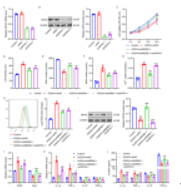
图3鼠双微体同源基因2通过谷胱甘肽过氧化物酶4调节氧糖剥夺结合血红素诱导的BV2小胶质细胞铁死亡和M1/M2极化
m6A修饰是调节RNA稳定性、剪接、加工、转录和翻译的最丰富的转录后修饰[7]。RNA结合蛋白,如IGF2BPs,已被报道以m6A依赖的方式增强鼠双微体同源基因2 mRNA的稳定性和表达[8,9]。因此,我们进一步研究了m6A修饰是否调节氧糖剥夺结合血红素诱导的BV2小胶质细胞中鼠双微体同源基因2 mRNA的稳定性和表达。如图4A-4G所示,氧糖剥夺结合血红素诱导鼠双微体同源基因2 m6A修饰并且增加WTAP表达。同时,WTAP介导鼠双微体同源基因2 m6A修饰,并通过IGF2BP1促进鼠双微体同源基因2 mRNA的稳定性和表达(图4H-4N)。此外,WTAP介导的鼠双微体同源基因2 m6A修饰影响氧糖剥夺结合血红素诱导的BV2小胶质细胞铁死亡和M1/M2极化(图5A-5J)。
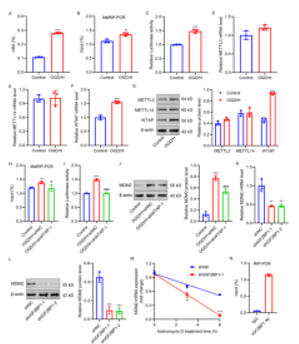
图4 WTAP诱导鼠双微体同源基因2 m6A修饰

图5 WTAP诱导的鼠双微体同源基因2 m6A修饰影响氧糖剥夺结合血红素诱导的BV2小胶质细胞铁死亡和M1/M2极化
最后,对鼠双微体同源基因2抑制剂可减轻与鼠双微体同源基因2 -谷胱甘肽过氧化物酶4调节轴相关的脑出血小鼠的神经功能受损进行了详细研究。如图6A-6E所示,与模型组相比,鼠双微体同源基因2抑制剂brigimadlin显著降低了NDS、逃逸潜伏期和靶区停留时间。脑出血显著降低GSH水平,增加MDA、亚铁离子、ROS含量(图6F-6I)。脑出血还增加了脑组织中iNOS、IL-1β、TNF-α的mRNA表达和分泌,降低了Arg-1、IL-10和TGF-的mRNA表达和分泌(图6J-6L)。然而,brigimadlin治疗逆转了上述脑出血的作用(图6A-6L)。另外,脑出血还显著诱导了WTAP和鼠双微体同源基因2的mRNA和蛋白质水平,抑制了谷胱甘肽过氧化物酶4的mRNA和蛋白质水平。brigimadlin处理后,WTAP和鼠双微体同源基因2的mRNA和蛋白表达水平未受影响,而谷胱甘肽过氧化物酶4水平恢复(图7A-7I)。这些结果表明,鼠双微体同源基因2诱导的谷胱甘肽过氧化物酶4泛素化在脑出血神经损伤的诱导中起关键作用,其与铁凋亡和氧化应激有关。
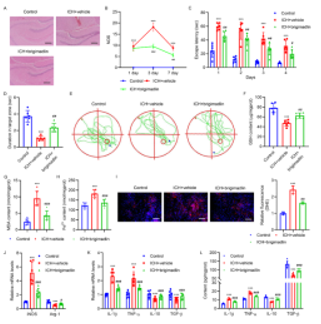
图6 Brigimadlin可减轻脑出血小鼠的空间记忆损害
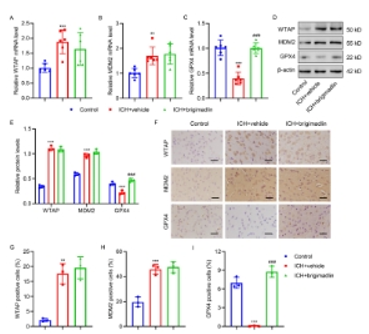
图7 小鼠脑出血后WTAP、鼠双微体同源基因2和谷胱甘肽过氧化物酶4的表达
总之,该研究提供了全面的体外和体内数据,证明干扰鼠双微体同源基因2可以减轻脑出血诱导的神经功能障碍和氧糖剥夺结合血红素诱导的脑小胶质细胞损伤,突出了其在治疗脑出血诱导的脑损伤方面的潜在应用。此外,鼠双微体同源基因2与谷胱甘肽过氧化物酶4相互作用,鼠双微体同源基因2通过促进其泛素化而下调谷胱甘肽过氧化物酶4蛋白水平,从而导致氧糖剥夺结合血红素诱导的小胶质细胞功能障碍。这些发现表明,靶向鼠双微体同源基因2和谷胱甘肽过氧化物酶4是一种很有前景的脑出血治疗策略。
当然该研究也存在一定性局限性。虽然在细胞和动物中都进行了实验,但该研究的一个局限性是缺乏对WTAP在脑出血小鼠铁死亡和炎症中的上游调节作用的体内动物实验验证。其次,该研究使用小鼠BV2小胶质细胞进行体外研究。BV2小胶质细胞是一种不能完全取代原代细胞的小胶质细胞系。选择原代培养的小胶质细胞进行体外体内研究,可能更能反映真实状态。此外,参与鼠双微体同源基因2表达调控的其他上游转录后或翻译后修饰尚不清楚。最后,是否其他关键形式的细胞死亡,如凋亡和焦亡,参与脑出血尚不清楚。这些局限性将在未来的研究中得到解决。
参考文献
1.Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother.2019;19(7):679-694.
2.Weimar C, Kleine-Borgmann J. Epidemiology, prognosis and prevention of non-traumatic intracerebral hemorrhage. Curr Pharm Design. 2017;23(15):2193-2196.
3.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720-731.
4.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355-369.
5.Lu J, Li H, Yu Z, et al. Cathepsin B as a key regulator of ferroptosis in microglia following intracerebral hemorrhage. Neurobiol Dis. 2024;194:106468.
6.Fei X, Dou Y, Yang Y, et al. Lipocalin-2 inhibition alleviates neural injury by microglia ferroptosis suppression after experimental intracerebral hemorrhage in mice via enhancing ferritin light chain expression. Biochim Biophys Acta Mol Basis Dis. 2024;1870(7):167435.
7.Jiang X, Liu B, Nie Z, et al. The role of m6A modification in the biological functions and diseases.Signal Transduct Target Ther. 2021;6(1):74.
8.Mu H, Cai S, Wang X, et al. RNA binding protein IGF2BP1 meditates oxidative stress-induced granulosa cell dysfunction by regulating MDM2 mRNA stability in an m6A-dependent manner.Redox Biol. 2022;57:102492.
9.Han P, Wei S, Wang H, et al. Licochalcone A decreases cancer cell proliferation and enhances ferroptosis in acute myeloid leukemia through suppressing the IGF2BP3/MDM2 cascade.Ann Hematol. 2024;103(11):4511-4524.






