NRR:中国上海交通大学付华林团队揭示出血性病理性轴突肿大与淀粉样化溶酶体不稳定性,是阿尔茨海默病中的一个重要缺陷
撰文:付华林
阿尔茨海默病是影响中国近千万人并给个人、家庭和社会带来沉重经济和精神负担的重大疾病,其重要病理特征包括老年斑、神经纤维缠结及神经营养不良。其中,神经营养不良包括轴突营养不良和树突营养不良[1]。既往研究已表明,老年斑的发生可能是由脑微动脉瘤破裂及血液淀粉样蛋白物质泄露而诱发形成[2],但是血液泄露如何影响阿尔茨海默病其他病理特征如神经营养不良缺乏相关研究。此外,Aβ及Tau磷酸化的在阿尔茨海默病中的相互关系以及它们对神经轴突营养不良的影响机制也是长期以来悬而未决的问题。这些问题对于阿尔茨海默病的预防及治疗至关重要。
中国上海交通大学电子信息与电气工程学院付华林团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Pathological axonal enlargement in connection with amyloidosis, lysosome destabilization, and bleeding is a major defect in Alzheimer’s disease”的研究。结果发现,Aβ不仅沉积于老年斑,还广泛存在于阿尔茨海默病患者脑部神经细胞的轴突中,其中Aβ沉积现象在脑部白质和灰质交界处的轴突束中尤其显著。阿尔茨海默病患者轴突普遍存在病理性扩张肿胀的现象,富含Aβ的阿尔茨海默病患者轴突直径约为对照者脑神经轴突的1.72倍。进一步研究发现,淀粉样化轴突还存在MAP2丢失、Tau磷酸化、弥散性溶酶体不稳定性以及存在出血性物质如ApoE,HBA,HbA1C和Hemin沉积等特征。溶酶体不稳定性在阿尔茨海默病组织神经细胞溶酶体中也清晰可见,富含Aβ的溶酶体直径约为不含Aβ溶酶体的2.23倍,这些溶酶体中不仅富含Aβ还同时伴随血源性物质如HBA甚至血管来源物质ACTA2和ColIV的共表达。在少数极端情况下,淀粉样化轴突中还可观察到轴突断裂,可能造成神经华勒氏变性的后果。这项研究表明,阿尔茨海默病神经细胞中除存在受到广泛研究的神经细胞胞体和突触缺陷外,轴突损伤和连接组破坏是阿尔茨海默病神经病变中一个非常关键的环节。付华林助理研究员为文章的第一作者及通讯作者。
阿尔茨海默病是一种多部位多发的淀粉样化的慢性神经退行性疾病。既往研究已表明,Aβ不仅沉积于老年斑,还沉积于阿尔茨海默病患者脑部的淀粉样血管,也存在于特殊的脑血管病变如微动脉瘤中。虽然作者既往研究以微动脉瘤破裂造成的血管内溶血性Aβ及其他血液血管蛋白泄露部分解释了老年斑的形态发生,但是还没有能够阐明溶血性Aβ及其他蛋白质如何和神经细胞发生相互作用而导致阿尔茨海默病患者失智的表型。此次研究采用了免疫组化染色和免疫荧光染色的方法分析了阿尔茨海默病患者前脑脑组织切片。共聚焦显微镜观察表明,Aβ淀粉样蛋白沉积还大量富集于阿尔茨海默病患者脑神经细胞的轴突及轴突束中(图1)。阿尔茨海默病患者脑部轴突束中大量富集Aβ,这个观察结果有一定创新性,且既往研究鲜见有类似报道。此外,阿尔茨海默病患者神经细胞轴突相比对照脑组织神经细胞轴突来说,其直径有显著增大,约是对照轴突直径的1.72倍,而且在相差显微镜下,淀粉样化轴突有更强反差颜色更暗,可以清晰识别。进一步分析了轴突淀粉样化对轴突分化标记MAP2的影响,结果显示阿尔茨海默病患者轴突中MAP2表达显著减少, MAP2表达在老年斑区域非常弱(图2)。MAP2减少现象在脑神经细胞轴突束中尤其明显(图2A),而且轴突中MAP2表达和轴突Aβ沉积及淀粉样化物质自发蓝色荧光强度呈明显的负相关(图2B和C)。
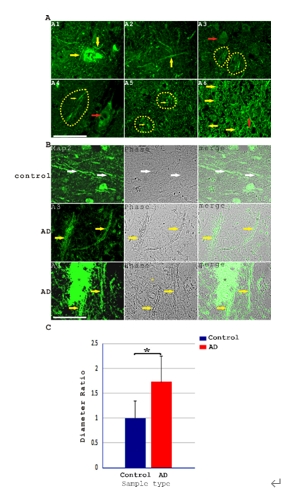
图1:轴突淀粉样化及异常肿胀是阿尔茨海默病的显著病理特征(图源:Fu et al., Neural Regen Res, 2026)

图2:淀粉样化轴突中MAP2水平显著下降(图源:Fu et al., Neural Regen Res, 2026)
轴突中MAP2因为何种原因而减少?作者的假设是某些异常的蛋白降解途径可能导致了MAP2的减少。细胞中的蛋白质降解途径主要包括泛素降解途径和溶酶体降解途径。这篇文章主要探讨了溶酶体途径异常在阿尔茨海默病脑组织中的表现。数据显示,大量Aβ阳性神经细胞中表现出溶酶体不稳定性,其特征包括溶酶体的膨胀、聚集以及弥散性的溶酶体标记染色(图3)。Aβ阳性溶酶体直径约是对照Aβ阴性溶酶体的2.23倍,Aβ阳性溶酶体的膨胀可以在单个溶酶体水平观察到。进一步的研究表明,溶酶体的不稳定性可能不完全归因于Aβ,因为阿尔茨海默病神经细胞溶酶体中还含有大量和脑出血相关的血浆或者血管蛋白,例如HBA,ACTA2和ColIV。阿尔茨海默病神经细胞中溶酶体不稳定性在神经细胞的多个部位都存在,除细胞体外,也存在于轴突及老年斑周边的神经营养不良结构中。阿尔茨海默病神经细胞轴突中同样存在脑出血相关标志物,如ApoE,HBA,HbA1C和Hemin。淀粉样化轴突肿胀可能和溶酶体的不稳定性及膨胀有关。溶酶体的膨胀和聚集的另一个体现是轴突中球状体(spheroid)的形成,轴突球状体中也富集Aβ及溶酶体相关标记如Sortilin 1的表达。在极少数情况下,作者还观察到轴突的断裂,可能和轴突中跨越较长距离的较大的球状体形成和破裂有关。

图3:Aβ在阿尔茨海默病神经元溶酶体中的富集和溶酶体的不稳定性如溶酶体膨胀、聚集、弥散性溶酶体Cathepsin D标记等特征密切相关(图源:Fu et al., Neural Regen Res, 2026)
长期以来,Aβ和Tau磷酸化都被作为阿尔茨海默病神经元退行性变化的标志,但是Aβ和Tau磷酸化有何关系并不明确。既往研究已表明,Aβ和Tau磷酸化可能都存在于神经元突触,和突触损伤相关[3]。本研究提出另一种解释,即Aβ和Tau磷酸化都和溶酶体不稳定性相关。数据表明,Tau磷酸化和溶酶体不稳定性关系密切。老年斑周边神经营养不良以及神经细胞缠结、神经纤维缠结、轴突中都能观察到弥散性溶酶体Lamp2标记和Tau磷酸化的共表达(图4),这些部位同时存在Aβ和Tau磷酸化的共表达。作者认为,溶酶体不稳定性可能就是Aβ和Tau磷酸化关键衔接点。
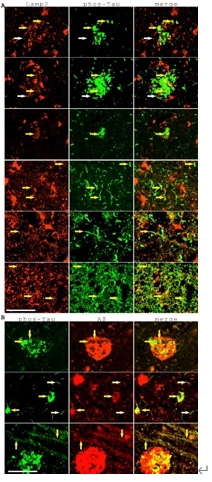
图4:Tau磷酸化和溶酶体不稳定性及Aβ表达密切相关,Tau磷酸化标记的老年斑周边神经营养不良以及神经细胞缠结、神经纤维缠结,轴突中都能观察到弥散性溶酶体Lamp2标记以及Aβ的表达(图源:Fu et al., Neural Regen Res, 2026)
此次实验结果提示,轴突的淀粉样化病变是阿尔茨海默病患者神经细胞的重要病理特征,轴突淀粉样病变和轴突病理性肿胀、球状体形成甚至轴突断裂相关。淀粉样化轴突呈现轴突去分化表型,例如MAP2的减少,MAP2的减少可能和病理性Aβ摄入引起的溶酶体不稳定性及溶酶体蛋白酶泄漏造成的不正常降解有关。阿尔茨海默病患者神经细胞溶酶体不仅含有大量Aβ,还包含大量和脑出血相关蛋白质,付华林等认为阿尔茨海默病患者神经细胞中Aβ主要可能是来自于血液淀粉样物质的慢性泄露,而不是来自于神经细胞本身。溶酶体不稳定性伴随的细胞毒性可能是造成阿尔茨海默病中神经细胞及轴突损伤的重要原因,也是Aβ和Tau磷酸化之间的关键衔接点。研究还指出,预防和治疗阿尔茨海默病首先应该预防和减少脑部血液泄露。另外,应设法减少对轴突的损伤,促进神经纤维再生和修复。虽然目前阿尔茨海默病治疗市场上已经存在药监局批准的针对Aβ的单克隆治疗性抗体如Lecanemab和Donanemab[4],这些药物能否有效降低神经细胞及轴突内的Aβ水平以减少轴突和连接组损伤,还需要进一步的深入观察和验证。在生物学理论上,提出溶酶体损伤泄露不见得会快速造成细胞死亡,细胞内可能存在复杂的对冲溶酶体损伤的调节机制,对这些调节机制的研究可能有助于对阿尔兹海默及其他溶酶体异常相关神经退行性疾病的认识,有助于研发新一代的阿尔茨海默病治疗药物。由于研究中分析的病理标本有限,未能对不同Braak阶段的阿尔茨海默病病理组织进行比较性研究,所观察到的神经细胞溶酶体及轴突病变表型可能还不够全面,有待进一步研究。
原文链接:https://doi.org/10.4103/NRR.NRR-D-24-01440
参考文献
[1] Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184-185.
[2] Fu H, Li J, Du P, et al. Senile plaques in Alzheimer's disease arise from Aβ- and Cathepsin D-enriched mixtures leaking out during intravascular haemolysis and microaneurysm rupture. FEBS Lett. 2023;597(7):1007-1040.
[3] Fein JA, Sokolow S, Miller CA, et al. Co-localization of amyloid beta and tau pathology in Alzheimer's disease synaptosomes. Am J Pathol. 2008;172(6):1683-1692.
[4] Terao I, Kodama W. Comparative efficacy, tolerability and acceptability of donanemab, lecanemab, aducanumab and lithium on cognitive function in mild cognitive impairment and Alzheimer's disease: A systematic review and network meta-analysis. Ageing Res Rev. 2024;94:102203.



