中国神经再生研究(英文版) ›› 2024, Vol. 19 ›› Issue (11): 2480-2487.doi: 10.4103/1673-5374.390966
Cav3.2通道调控脑缺血再灌注损伤:有前途的干预靶点
Cav3.2 channel regulates cerebral ischemia/reperfusion injury: a promising target for intervention
Feibiao Dai1, 2, 3, #, Chengyun Hu1, 2, 3, #, Xue Li1, 2, 3, #, Zhetao Zhang3, 4, Hongtao Wang2, 3, Wanjun Zhou2, 3, Jiawu Wang2, 3, Qingtian Geng2, 3, *, Yongfei Dong3, 5, *, Chaoliang Tang2, 3, *
- 1Graduate School, Wannan Medical College, Wuhu, Anhui Province, China; 2Department of Anesthesiology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui Province, China; 3Core Facility Center for Medical Sciences, The First Affiliated Hospital of USTC (Anhui Provincial Hospital), Hefei, Anhui Province, China; 4Department of Pharmacy, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui Province, China; 5Department of Neurosurgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui Province, China
摘要:
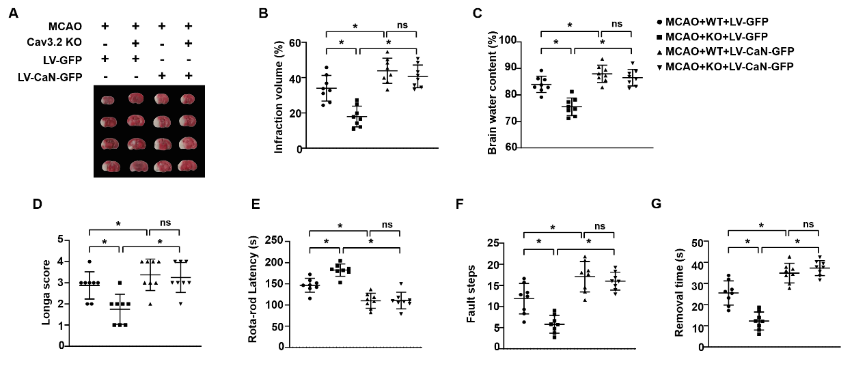
在脑缺血再灌注损伤中,钙流入大脑神经元引发神经元死亡,且多种钙通道参与了脑缺血再灌注损伤。Cav3.2通道是T型钙通道的主要亚型,有研究发现T型钙通道阻滞剂匹莫齐德和米贝拉地尔可预防脑缺血再灌注损伤诱导的脑损伤,但是Cav3.2通道在脑缺血再灌注损伤中的作用尚未阐明。为此,实验分别以大脑中动脉阻塞诱导小鼠脑缺血再灌注损伤以及高糖缺氧/复氧诱导原代海马神经元以构建体内外脑缺血再灌注损伤模型,发现损伤的海马组织和原代海马神经元中Cav3.2表达显著上调。实验进一步建立Cav3.2基因敲除小鼠脑缺血再灌注损伤模型,发现Cav3.2敲除可显著减轻脑缺血再灌注损伤所致脑梗死体积、脑含水量以及神经功能障碍。此外,Cav3.2敲除还减弱了脑缺血再灌注损伤诱导的氧化应激、炎症反应和神经元凋亡。而在Cav3.2敲除小鼠海马中过表达钙调神经磷酸酶可抵消Cav3.2敲除对脑缺血再灌注损伤的保护作用。上述结果表明,Cav3.2敲除介导的神经保护功能是由钙调神经磷酸酶/激活T细胞核因子3信号传导介导的。这项研究表明,Cav3.2可能是脑缺血再灌注损伤的一个有前途的干预靶点。
https://orcid.org/0000-0002-6873-7821 (Qingtian Geng); https://orcid.org/0000-0002-1740-1375 (Yongfei Dong);
https://orcid.org/0000-0002-1936-028X (Chaoliang Tang)