NRR:中国昆明医科大学第二附属医院燕晋媛和赵宁辉团队揭示壳聚糖可作为治疗帕金森病潜在药物的作用机制
#br#
撰文:王银莹,陈蓉莎,燕晋媛
#br#
帕金森病的特点是黑质多巴胺神经元变性以及α-突触核蛋白积累,并伴有运动和非运动症状等。2022年世界卫生组织报告称,帕金森病患者的数量在过去25年中翻了一番,帕金森病引起的残疾和死亡人口比例高于其他任何神经系统疾病 [1]。目前帕金森病的治疗包括药物治疗和深部脑刺激。然而,这些药物和疗法并不是所有人都能获得或负担得起。
多糖存在于微生物、藻类、植物和动物中,具有丰富的生物活性。纤维素和淀粉等多糖被降解和吸收,它们的代谢产物调节宿主的代谢和病理生理。而不能被水解和吸收及不能消化的多糖可能直接通过多糖受体起作用或被宿主肠道微生物代谢 [2]。在细胞模型中发现壳聚糖通过减少线粒体损伤和活性氧的产生,抑制细胞凋亡;抑制线粒体功能障碍、核凝聚和DNA断裂保护神经细胞 [3-5]。此外,壳聚糖由于其强渗透性、黏液粘附性、生物相容性和可降解性,可以作为纳米颗粒将药物输送到中枢神经系统 [6-11]。这些研究表明,壳聚糖可以在细胞模型中保护神经元死亡,或在小鼠模型中作为纳米颗粒转移药物。然而,壳聚糖并不能血脑屏障,不能被宿主消化,因此其在帕金森病中的作用还有待探讨。
最近,来自中国昆明医科大学第二附属医院燕晋媛、赵宁辉团队在《中国神经再生研究(英文)》(Neural Regeneration Research)上发表了题为“Chitosan alleviates symptoms of Parkinson’s disease by reducing
acetate levels, which decreases inflammation and promotes repair of the
intestinal barrier and blood– brain barrier”的文章。该研究发现壳聚糖显著降低肠道微生物多样性及其代谢产物短链脂肪酸乙酸盐,并抑制肠道中乙酸盐激活的PPARD/AMPK信号通路,减轻炎症,修复肠道屏障和血脑屏障进而缓解帕金森病。这些发现提示壳聚糖可作为潜在的预防和治疗帕金森病的药物。
世界卫生组织报告显示,帕金森病的致残和致死率已远高于其他神经系统疾病 [1]。帕金森病已经成为影响国民寿命的三大杀手之一。然而药物疗效局限,在低收入和中等收入国家,脑深部电刺激并非所有人可负担,因此,找寻疗效更好、适用范围更广、不良反应更少的药物是目前帕金森病基础研究及临床研究中亟待解决的问题。有研究提示,壳聚糖在神经退行性疾病中具有保护作用。但是在帕金森病小鼠中,壳聚糖是如何实现神经保护作用的?壳聚糖作为潜在的帕金森病的神经保护剂值得深入研究。因此,实验首先检测壳聚糖治疗是否对帕金森病具有神经保护作用。结果发现壳聚糖可有效改善多巴胺能神经元的损伤、神经递质多巴胺的释放以及运动功能障碍(图1)。这些结果证明壳聚糖可以缓解或治疗帕金森病。由于壳聚糖是大分子多糖不能通过血脑屏障直接作用于中枢神经系统,随后研究发现壳聚糖的神经保护作用跟肠道微生物代谢产物短链脂肪酸有关,壳聚糖可以改变肠道微生物丰度并降低短链脂肪酸含量。以往的研究显示短链脂肪酸与肠屏障受损有关 [12, 13],既然短链脂肪酸可以修复肠道屏障,壳聚糖是否通过调控短链脂肪酸抵抗帕金森病呢?因此燕晋媛和赵宁辉团队检测壳聚糖对肠道和血脑屏障的影响,结果发现壳聚糖可有效改善肠屏障和血脑屏障损伤(图2)。然而又是何种短链脂肪酸起作用的呢,实验进一步通过回补不同短链脂肪酸验证其在帕金森病中的作用。结果发现乙酸盐不仅损伤肠道屏障功能,还加重了外周及中枢的炎症反应。以上的研究结果证明壳聚糖是通过减少乙酸盐减轻炎症反应来改善肠屏障和血脑屏障。进一步通过结肠转录组测序、体内及体外实验发现:乙酸盐可能是通过结合肠道PPARD受体调控 PPAR/AMPK信号通路从而减轻炎症反应并缓解帕金森病(图3)。这些发现指出了壳聚糖通过减少乙酸盐抑制炎症修复肠道屏障和血脑屏障的分子机制,为临床治疗和预防帕金森病提供新的思路。
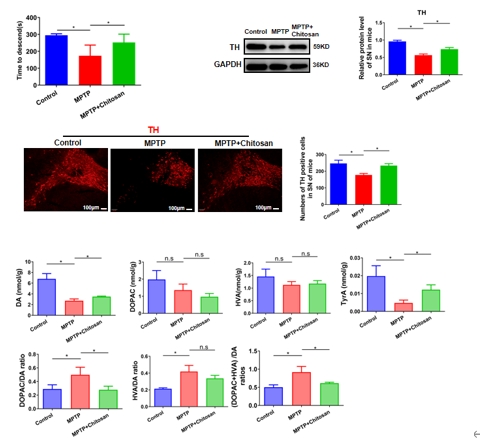
图1 壳聚糖可缓解1-甲基-4-苯基-1,2,3,6-四氢吡啶诱导的帕金森病模型小鼠的运动功能障碍,提高多巴胺神经元的存活率(图源:Wang et al., Neural Regen Res, 2026)
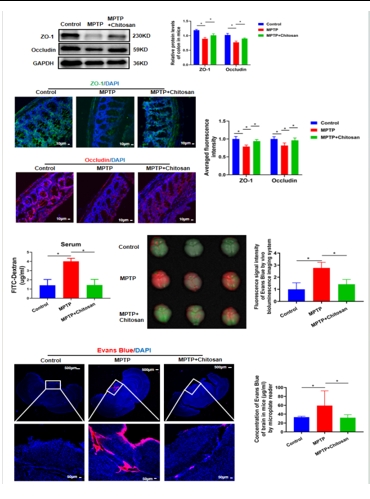
图2壳聚糖可修复帕金森病模型小鼠受损的肠脑屏障和血脑屏障(图源: Wang et al., Neural Regen Res, 2026)
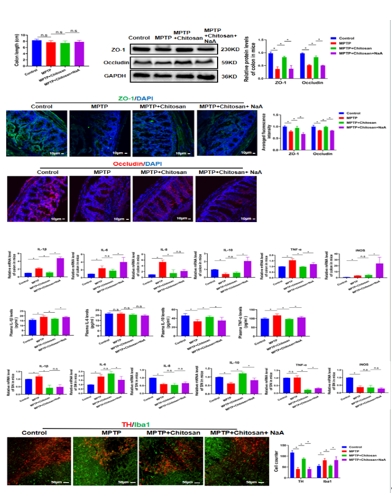
图3醋酸盐破坏壳聚糖修复的肠道屏障,使结肠、血浆和黑质的炎症反应升高,最终促进帕金森病模型中小胶质细胞的活化(图源:Wang et al., Neural Regen Res, 2026)
#br#
综上所述,这些发现提示壳聚糖通过减少短链脂肪酸来抑制炎症并修复肠道屏障和血脑屏障的分子机制,提供治疗帕金森病的新方法。研究发现,1-甲基-4-苯基-1,2,3,6-四氢吡啶处理的小鼠血脑屏障受损,壳聚糖治疗后的肠道乙酸减少,抑制促炎因子并修复血脑屏障完整性,但不改变血浆或大脑中的短链脂肪酸;然而,补充乙酸盐后,小鼠表现出炎症升高、肠屏障损伤和帕金森病症状加重。短链脂肪酸可以穿过血脑屏障,以维持中枢神经系统的完整性和功能。然而作者发现壳聚糖治疗组中脑内SCFAs与MPTP诱导的帕金森病小鼠没有差异,因此推断短链脂肪酸主要作用部位是肠道。此项研究结果将为缓解帕金森病提供一定的基础。然而这项研究仍有一些局限性。首先,作者检测微生物,但没有聚焦到具体哪种肠道微生物在帕金森病中有作用。其次,微生物代谢产物种类繁多,本研究作者重点研究了短链脂肪酸,但是壳聚糖还可能通过其他微生物代谢产物缓解帕金森病。最后,壳聚糖在调节肠道微生物和短链脂肪酸平衡方面的作用仍然值得进一步研究。
原文链接:https://doi.org/10.4103/NRR.NRR-D-23-01511
#br#
参考文献
[1] Parkinson disease: a public health approach. Technical brief. 2022. Geneva: World Health Organization.
[2] Ahmadi S, Mainali R, Nagpal R, et al. Dietary polysaccharides in the amelioration of gut microbiome dysbiosis and metabolic diseases. Obes Control Ther. 2017;4(3):10.15226/12374-18354/15224/15222/00140.
[3] Wang Y, Chen R, Yang Z, et al. Protective effects of polysaccharides in neurodegenerative diseases. Front Aging Neurosci. 2022;14:917629.
[4] Wang X, Miao J, Yan C, et al. Chitosan attenuates dibutyltin-induced apoptosis in PC12 cells through inhibition of the mitochondria-dependent pathway. Carbohydr Polym. 2016;151:996-1005.
[5] Manigandan V, Nataraj J, Karthik R, et al. Low molecular weight sulfated chitosan: neuroprotective effect on rotenone-induced in vitro Parkinson's disease. Neurotox Res. 2019;35(3):505-515.
[6] Yu S, Xu X, Feng J, et al. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282-293.
[7] Rassu G, Soddu E, Cossu M, et al. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J Drug Deliv Sci Technol. 2016;32:77-87.
[8] Bhattamisra SK, Shak AT, Xi LW, et al. Nose to brain delivery of rotigotine loaded chitosan nanoparticles in human SH-SY5Y neuroblastoma cells and animal model of Parkinson's disease. Int J Pharm. 2020;579:119148.
[9] Sardoiwala MN, Karmakar S, Choudhury SR. Chitosan nanocarrier for FTY720 enhanced delivery retards Parkinson's disease via PP2A-EzH2 signaling in vitro and ex vivo. Carbohydr Polym. 2021;254:117435.
[10] Xue Y, Wang N, Zeng Z, et al. Neuroprotective effect of chitosan nanoparticle gene delivery system grafted with acteoside (ACT) in Parkinson’s disease models. J Mater Sci Technol. 2020;43:197-207.
[11] Nehal N, Nabi B, Rehman S, et al. Chitosan coated synergistically engineered nanoemulsion of Ropinirole and nigella oil in the management of Parkinson's disease: Formulation perspective and In vitro and In vivo assessment. Int J Biol Macromol. 2021;167:605-619.
[12] Yang X, Ai P, He X, et al. Parkinson's disease is associated with impaired gut-blood barrier for short-chain fatty acids. Mov Disord. 2022;37(8):1634-1643.
[13] Wang J, Zhang C, Guo C, et al. Chitosan ameliorates DSS-induced ulcerative colitis mice by enhancing intestinal barrier function and improving microflora. Int J Mol Sci. 2019;20(22):5751.


